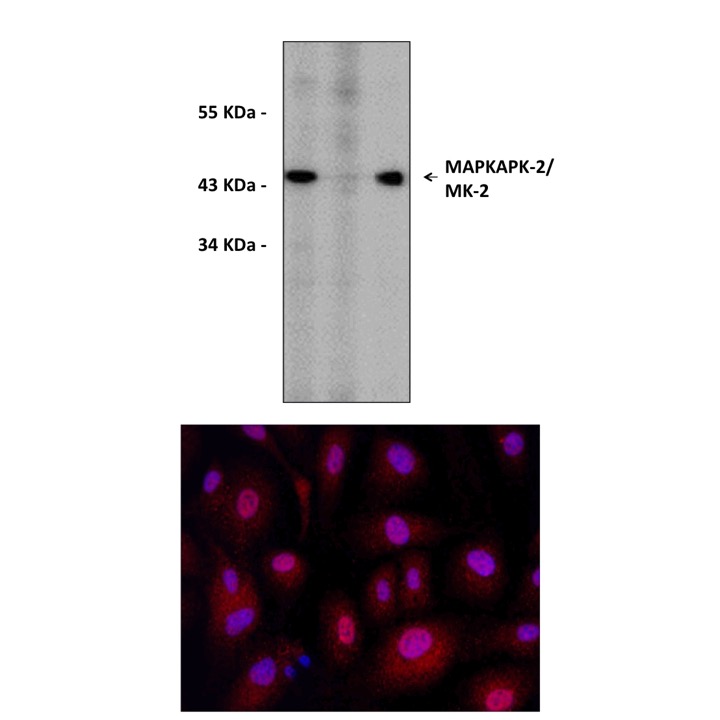
Anti-MK2: Rabbit MAPKAPK-2/MK-2 Antibody |
 |
BACKGROUND Mitogen-activated protein kinases, including ERK1/ERK2, JNK/SAPK, and p38/RK, are important signal transducing molecules for control of gene expression, cell proliferation, and apoptosis. In response to cellular stresses, such as heat or osmotic shock, bacterial lipopolysaccharide, proinflammatory cytokines, and tumor necrosis factor-alpha, p38 MAP kinase is activated by its upstream kinases MKK3 and MKK6. Activated p38 phosphorylates MAPKAPK-2, MAPKAPK-3, PRAK, MNK1/2, MSK1, and transcription factors ATF2, CHOP/GADD153, Elk-1, and MEF2C. MAPKAPK-2 is activated in vivo only by p38/p40/RK. Multiple residues of MAPKAPK-2 are phosphorylated by p38 MAPK. Phosphorylation at Thr222, Ser272 and Thr334 appears to be essential for the activity of MAPKAPK-2.1 The p38 C-terminal regulatory domain contains a bipartite nuclear localization signal and a nuclear export signal. Following phosphorylation of MAPKAPK-2, nuclear p38 was exported to the cytoplasm in a complex with MAPKAPK-2. The p38 activators MKK3 and MKK6 were present in both the nucleus and the cytoplasm, consistent with a role in activating p38 in the nucleus. Thus, MAPKAPK-2 serves both as an effector of p38 by phosphorylating substrates and as a determinant of cellular localization of p38. Nuclear export of p38 and MAPKAP kinase-2 may permit them to phosphorylate substrates in the cytoplasm.2
Mice that lack MAPKAPK-2 show increased stress resistance and survive bacterial lipopolysaccharide-induced endotoxic shock due to a 90% reduction in the production of tumor necrosis factor-alpha.3 MAPKAPK-2 is in the nucleus of unstimulated cells and moves rapidly to the cytoplasm after stimulation. In the nucleus, MAPKAPK-2 contributes to the phosphorylation of CREB at Ser133 and may regulate its ability to activate transcription in response to cAMP, Ca2+, and nerve growth factor. MAPKAPK-2 phosphorylates serum response factor at Ser103 both in vivo and in vitro in response to tumor-promoting and stress inducing stimuli. Both MAPKAPK-2 and MAPKAPK-3 interact with basic helix-loop-helix transcription factor E47 in vivo and phosphorylate E47 in vitro, suggesting that they are regulators of E47 activity and E47-dependent gene expression. In the cytoplasm, MAPKAPK-2 phosphorylates small heat shock protein HSP25/HSP27 and lymphocyte-specific protein LSP-1, both F-actin-binding proteins. Other substrates of MAPKAPK-2 include glycogen synthase, tyrosine hydroxylase, and 5-lipoxygenase. MAPKAPK-2 is directly responsible for phosphorylating Cdc25B and C and maintaining the G1, S, and G2/M checkpoints in response to UV-induced DNA damage.4 In addition, MAPKAPK-2 can also phosphorylate HDM2 on serine 157 and 166. Phosphorylation of these sites appears to contribute to the activation of HDM2 and therefore a reduction in p53 stability, and may play a role in moderating the extent and duration of a stress-induced induction of the p53 response.5
REFERENCES
1. Ben-Levy, R. et al: EMBO J. 14:5920-30, 1995
2. Ben-Levy, R. et al: Curr. Biol. 8:1049-57, 1998
3. Hegen, M. et al: J. Immunol. 177:913-17, 2006
4. Manke, I.A. et al: Mol. Cell 17:37-48, 2005
5. Weber, H.O. et al: Oncogene 24:1964-75, 2005
2. Ben-Levy, R. et al: Curr. Biol. 8:1049-57, 1998
3. Hegen, M. et al: J. Immunol. 177:913-17, 2006
4. Manke, I.A. et al: Mol. Cell 17:37-48, 2005
5. Weber, H.O. et al: Oncogene 24:1964-75, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1296 |
Antigen: | Synthetic peptide corresponding to amino acids 1-14 of human MAPKAPK2. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:500-1:2000 IP n/d IHC n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 45 kDa |
Specificity/Sensitivity: | Detects endogenous MK2 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой