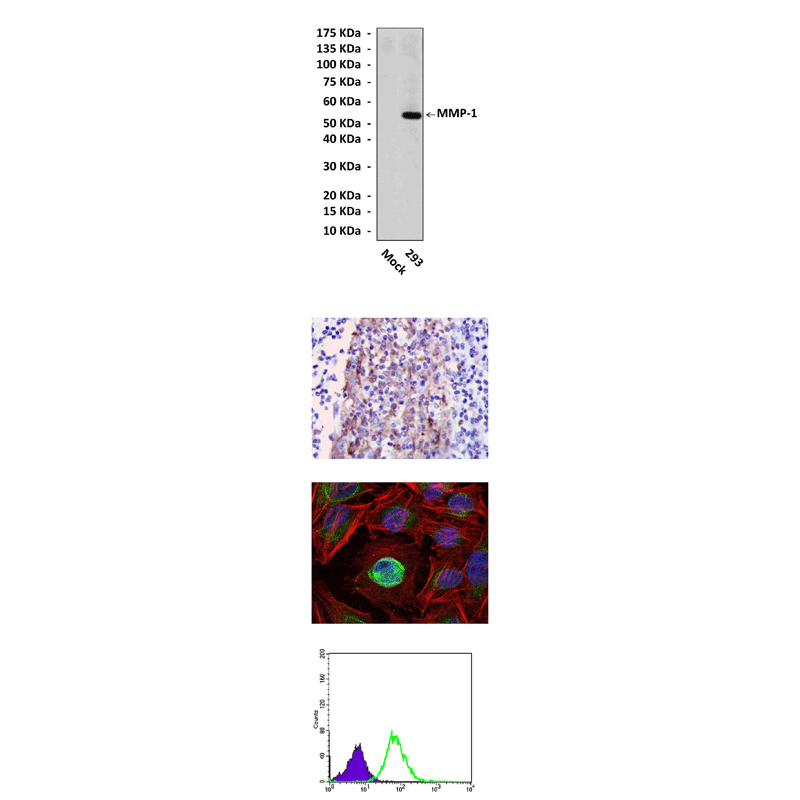
Anti-MMP-1: Mouse MMP-1 Antibody |
 |
BACKGROUND The matrix metalloproteinases (MMPs) are a family of zinc-containing endopeptidases that play a key role in both physiological and pathological tissue remodeling. 21 different human MMPs have been identified. These enzymes are classified according to their structure and substrate specificity into four major subgroups: the interstitial collagenases, gelatinases, stromelysins and membrane-type MMPs (MT-MMPs). MMPs regulate a variety of normal cell functions and host defense mechanisms. They are also associated with the tumorigenic process. MMPs degrade the extracellular matrix, promoting tumor invasion and metastasis. Collagenase-1 (MMP-1), collagenase-2 (MMP-8) and collagenase-3 (MMP-13) are the principal secreted neutral proteinases for the degradation of collagens. They all cleave type I, II and III collagens at a specific site generating 3/4 N-terminal and ¼ C-terminal fragments, which rapidly denature at physiological temperatures and become susceptible to degradation by other MMPs e.g. gelatinases.1
Human fibroblast collagenase (MMP-1) was the first vertebrate collagenase purified as a protein and cloned as a cDNA, and is considered the prototype for all the interstitial collagenases. It is synthesized as a zymogen where N-terminal residues are removed by proteolysis and shares with other MMPs a catalytic domain and a carboxyl terminal domain with sequence similarity to hemopexin. Importantly, MMP-1 should be considered a multifunctional molecule since it participates not only in the turnover of collagen fibrils in the extracellular space but also in the cleavage of a number of non-matrix substrates and cell surface molecules suggesting a role in the regulation of cellular behaviour.2 The release of MMP-1 by endothelial cells is crucial for angiogenesis, and cleavage of collagen type I, a substrate of MMP-1, is required for endothelial cell invasion into the ECM and for vessel formation in vivo. MMP-1 was shown to specifically degrade insulin-like growth factor (IGF) binding proteins 2, 3 and 5, fibroblast growth factor (FGF) binding protein and transforming growth factor (TGF)- binding protein and release IGF, FGF and TGF- . Furthermore, an extensive body of evidence indicates that MMP-1 plays an important role in diverse physiologic processes such as development, tissue morphogenesis, and wound repair. Likewise, it seems to be implicated in a variety of human diseases including cancer, rheumatoid arthritis, pulmonary emphysema and fibrotic disorders, suggesting that its inhibition or stimulation may open therapeutic avenues. Elevated MMP-1 expression correlates with invasiveness of colorectal cancer and cutaneous melanoma, and is associated with lymph node metastasis in stage IB cervical cancer and peritoneal metastasis in gastric cancer. Animal studies have suggested that overexpression of MMP-1 protein has a role in initiating mammary tumorigenesis by degrading stroma and releasing growth factors and other mitogens for epithelial cellsECM degradation by MMPs including MMP-1 was also shown to perturb cell-cell and cell-ECM interactions and disassociate cells from ECM, leading to increased cell division, decreased apoptosis and tumorigenesis.3
Human fibroblast collagenase (MMP-1) was the first vertebrate collagenase purified as a protein and cloned as a cDNA, and is considered the prototype for all the interstitial collagenases. It is synthesized as a zymogen where N-terminal residues are removed by proteolysis and shares with other MMPs a catalytic domain and a carboxyl terminal domain with sequence similarity to hemopexin. Importantly, MMP-1 should be considered a multifunctional molecule since it participates not only in the turnover of collagen fibrils in the extracellular space but also in the cleavage of a number of non-matrix substrates and cell surface molecules suggesting a role in the regulation of cellular behaviour.2 The release of MMP-1 by endothelial cells is crucial for angiogenesis, and cleavage of collagen type I, a substrate of MMP-1, is required for endothelial cell invasion into the ECM and for vessel formation in vivo. MMP-1 was shown to specifically degrade insulin-like growth factor (IGF) binding proteins 2, 3 and 5, fibroblast growth factor (FGF) binding protein and transforming growth factor (TGF)- binding protein and release IGF, FGF and TGF- . Furthermore, an extensive body of evidence indicates that MMP-1 plays an important role in diverse physiologic processes such as development, tissue morphogenesis, and wound repair. Likewise, it seems to be implicated in a variety of human diseases including cancer, rheumatoid arthritis, pulmonary emphysema and fibrotic disorders, suggesting that its inhibition or stimulation may open therapeutic avenues. Elevated MMP-1 expression correlates with invasiveness of colorectal cancer and cutaneous melanoma, and is associated with lymph node metastasis in stage IB cervical cancer and peritoneal metastasis in gastric cancer. Animal studies have suggested that overexpression of MMP-1 protein has a role in initiating mammary tumorigenesis by degrading stroma and releasing growth factors and other mitogens for epithelial cellsECM degradation by MMPs including MMP-1 was also shown to perturb cell-cell and cell-ECM interactions and disassociate cells from ECM, leading to increased cell division, decreased apoptosis and tumorigenesis.3
REFERENCES
1. Malemud, C.J.: Front.in Biosci. 11:1696-701, 2006
2. Seiki, M.: Curr. Opin. Cell Biol. 14:624-32, 2002
3. Poola, I. et al: Nat. Med. 11:481-3, 2005
2. Seiki, M.: Curr. Opin. Cell Biol. 14:624-32, 2002
3. Poola, I. et al: Nat. Med. 11:481-3, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10422 |
Antigen: | Raised against recombinant human MMP-1 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:25 - 1:50 IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 54 kDa |
Specificity/Sensitivity: | Detects MMP-1 proteins in cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой