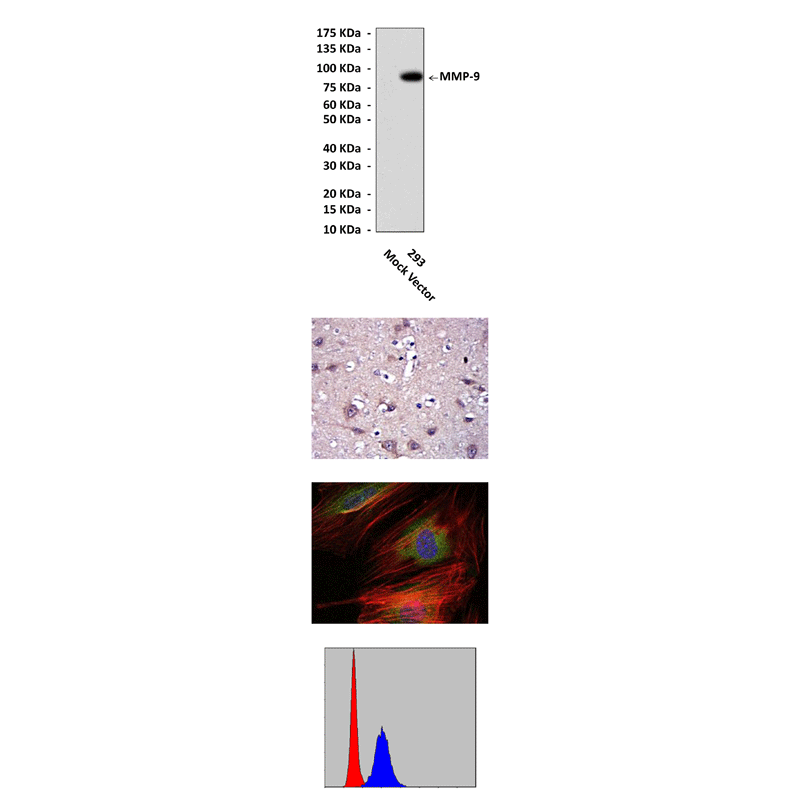
Anti-MMP-9: Mouse MMP-9 Antibody |
 |
BACKGROUND MMP-9 is a Zn+2 dependent endopeptidase, synthesized and secreted in monomeric form as zymogen. The structure is almost similar to MMP2. MMP-9 is first synthesized as inactive proenzyme or zymogens. Activation of proMMP-9 is mediated by plasminogen activator/plasmin (PA/plasmin) system. The regulation of MMP-9 activity is also controlled through TIMP-3. MMP-9 expression is regulated by several cytokines and growth factors, including interleukins, interferons, EGF, NGF, basic FGF, VEGF, PDGF, TNF-alpha, TGF-beta, the extracellular matrix metalloproteinase inducer EMMPRIN and also osteopontin.1 Many of these stimuli induce the expression and/or activation of c-fos and c-jun proto-oncogene products, which heterodimerize and bind activator protein-1 (AP-1) sites within of MMP9 gene promoters. Primary function of MMP-9 is degradation of proteins in the extracellular matrix. It proteolytically digests decorin, elastin, fibrillin, laminin, gelatin (denatured collagen), and types IV, V, XI and XVI collagen and also activates growth factors like proTGF-beta and proTNF-alpha. Physiologically, MMP-9 in coordination with other MMPs, play a role in normal tissue remodeling events such as neurite gowth, embryonic development, angiogenesis, ovulation, mammary gland involution and wound healing. MMP-9 with other MMPs is also involved in osteoblastic bone formation and/or inhibits osteoclastic bone resorption.2
Elevated expression of MMP-9, along with MMP-2 is usually seen in invasive and highly tumorigenic cancers.3 MMPs promote tumor progression and metastasis in invasive cancers by degradation of the ECM (ExtraCellular Matrix), which consists of two main components: Basement membranes and interstitial connective tissue. Though ECM comprises of many proteins (laminin-5, proteoglycans, entactin, osteonectin) collagen IV is the major element. MMP-2 & MMP-9 efficiently degrade collagen IV and laminin-5 thereby, assisting the metastatic cancerous cells to pass through the basement membrane. The degradation of ECM not only assists migration of metastatic cancerous cells, but also allows enhanced tumor growth by providing necessary space. Further, it is noteworthy that the ratio of active to latent form of MMP-9 increased with tumor progression in invasive cancers. MMP-9, with its family members also promotes angiogenesis (a critical process required for tumor cell survival) by degrading the vascular basement membrane interstitium and also by releasing sequestered VEGF, which is a well know angiogenic molecule. Localization of MMP9 to the cell surface is required to promote tumor invasion and angiogenesis.4
Elevated expression of MMP-9, along with MMP-2 is usually seen in invasive and highly tumorigenic cancers.3 MMPs promote tumor progression and metastasis in invasive cancers by degradation of the ECM (ExtraCellular Matrix), which consists of two main components: Basement membranes and interstitial connective tissue. Though ECM comprises of many proteins (laminin-5, proteoglycans, entactin, osteonectin) collagen IV is the major element. MMP-2 & MMP-9 efficiently degrade collagen IV and laminin-5 thereby, assisting the metastatic cancerous cells to pass through the basement membrane. The degradation of ECM not only assists migration of metastatic cancerous cells, but also allows enhanced tumor growth by providing necessary space. Further, it is noteworthy that the ratio of active to latent form of MMP-9 increased with tumor progression in invasive cancers. MMP-9, with its family members also promotes angiogenesis (a critical process required for tumor cell survival) by degrading the vascular basement membrane interstitium and also by releasing sequestered VEGF, which is a well know angiogenic molecule. Localization of MMP9 to the cell surface is required to promote tumor invasion and angiogenesis.4
REFERENCES
1. Atkinson, J.J. & Senior, R.M.: Am J Respir Cell Mol Biol. 28:12-24, 2003
2. Ishibashi, O. et al: Life Sci. 79:1657-60, 2006
3. Himelstein, B.P. et al: Invasion Metastasis. 14:246-58, 1994-1995
4. Vu, T.H. et al: Cell 93:411-22, 1998
2. Ishibashi, O. et al: Life Sci. 79:1657-60, 2006
3. Himelstein, B.P. et al: Invasion Metastasis. 14:246-58, 1994-1995
4. Vu, T.H. et al: Cell 93:411-22, 1998
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10408 |
Antigen: | Raised against recombinant human MMP-9 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 92 kDa |
Specificity/Sensitivity: | Detects MMP-9 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой