Anti-Myoglobin: Mouse Myoglobin Antibody |
 |
BACKGROUND Myoglobin is an extremely compact heme protein (MW ~ 17.8 kDa). It is found primarily in cardiac and red skeletal muscles, functions in the storage of oxygen and facilitates the transport of oxygen to the mitochondria for oxidative phosphorylation. Myoglobin is closely related to hemoglobin, which consists of four myoglobin-like subunits that form a tetramer and are responsible for carrying oxygen in blood. Myoglobin is a water-soluble globular protein of ~150 amino acids. The tertiary structure is composed of eight alpha-helices joined by short non-helical regions. The helices provide a rigid structural framework for the heme pocket. A heme group is bound in a hydrophobic cleft in the protein, and is key to the function of myoglobin: it is to the heme that oxygen binds. The heme itself consists of an organic ring known as protoporphyrin that surrounds an iron atom. The iron is ligated to four nitrogens of the protoporphyrin, as well as to a histidine side-chain of myoglobin which tethers the heme in the hydrophobic pocket. This leaves a sixth ligation position, on the side of the heme plane opposite (distal) to the histidine, available for the binding of oxygen. Interactions with residues near the oxygen binding site serve to stabilize bound oxygen, as well as interfere with CO binding. The interior and exterior of myoglobin are well-distinguished by hydrophobic and hydrophilic side groups. The structure and dynamics of the heme, and its surrounding environment, play a critical role in the ability of myoglobin to reversibly bind oxygen and resist carbon monoxide binding (the latter is catastrophic, since CO binds irreversibly to the heme, making it inaccessible to O2).1 The heme environment in myoglobin confers some protection against carbon monoxide poisoning. Because heme naturally binds CO 25, 000-times more strongly than it binds O2, carbon monoxide asphyxiates cells from within by blocking oxygen uptake (cyanide poisoning occurs in a similar fashion, binding to the heme of the electron transport protein cytochrome c). However, the so-called distal histidine of myoglobin, on the oxygen-binding heme face, forces CO (and O2) to bind at an angle to the heme, and is believed to be responsible for reducing by two orders of magnitude the affinity for CO. While O2 readily takes the conformation enforced by the distal histidine, CO does not.2 When a muscle is exercised, it uses up available oxygen. Myoglobin has oxygen bound to it, thus providing an extra reserve of oxygen so that the muscle can maintain a high level of activity for a longer period of time. When muscle is damaged, the myoglobin is released into the bloodstream. It is filtered out of the bloodstream by the kidneys, and eliminated in urine. In large quantities, myoglobin can damage the kidney and break down into toxic compounds, causing kidney failure. In humans, blood-borne cardiac myoglobin can serve as a biomarker of heart attack, since blood myoglobin levels rise in two to three hours following muscle injury.3
REFERENCES
1. George, A. et al: J. Exp. Biol. 207: 3441–6, 2004
2. Brunori, M. & Gibson, Q.H.: EMBO Rep. 2:676–9, 2001
3. Naka, T. et al: Critical Care 9: R90–5, 2005
2. Brunori, M. & Gibson, Q.H.: EMBO Rep. 2:676–9, 2001
3. Naka, T. et al: Critical Care 9: R90–5, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10314 |
Antigen: | Raised against recombinant human myoglobin fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 - 1:200 ICC n/d FACS n/d |
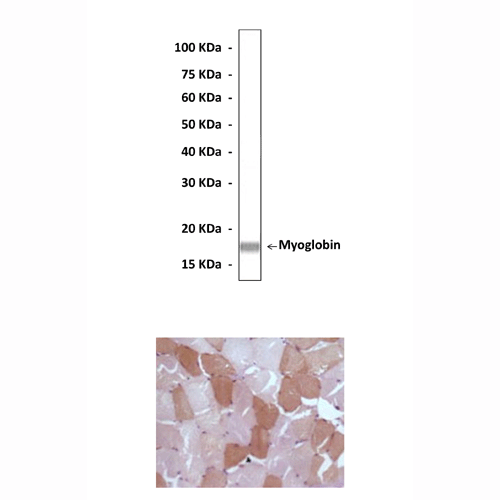
Predicted Molecular Weight of protein: | 17.8 kDa |
Specificity/Sensitivity: | Detects endogenous myoglobin proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой