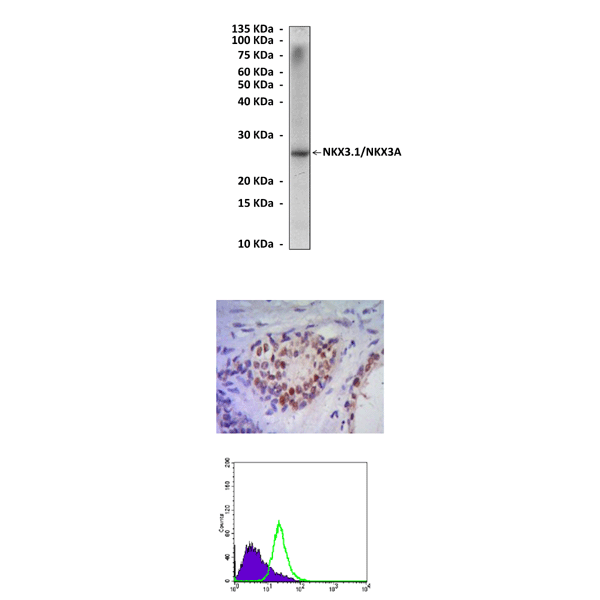
Anti-NKX3.1/NKX3A: Mouse NKX3.1/NKX3A Antibody |
 |
BACKGROUND Members of the NK subfamily of homeobox genes have been implicated in processes of cell fate specification and organogenesis in many species, including Drosophila, in which they were first identified. Molecular genetic analyses in Drosophila showed that NK-3 corresponded to the bagpipe gene, which is required for visceral mesoderm development, whereas NK-2 corresponded to tinman, which is required for cardiac mesoderm. In amniotes, these functional relationships appear to be conserved among Nkx homologs, although the specific functions are parceled out between two or more homologs for each NK ancestor. Thus, two NK-3 class genes are known in amniotes, corresponding to Nkx3.1 and Nkx3.2 (also known as Bapx1), with their overall patterns of gene expression during development well-conserved. A third Nkx3 family member has been identified in urodele amphibians, which contain a clear ortholog of Nkx3.2, but not of Nkx3.1; instead, a novel Nkx3.3 gene has been defined that has not been identified in higher vertebrates.1
The Nkx3.1 homeobox gene is a key regulator of prostatic epithelial differentiation, while its loss-of-function in mice has been implicated in prostate cancer initiation. In particular, Nkx3.1 null mutant mice display abnormal prostatic differentiation as well as epithelial hyperplasia and dysplasia prior to 1 year of age. Human NKX3.1 maps to chromosome 8p21, which frequently undergoes loss of heterozygosity at early stages of prostate carcinogenesis. Although NKX3.1 is not mutated in prostate cancer, it undergoes epigenetic inactivation through loss of protein expression in human prostate cancer and in mouse models.2 It was shown that PTEN loss causes reduced NKX3.1 expression in both murine and human prostate cancers. Restoration of Nkx3.1 expression in vivo in Pten null epithelium leads to decreased cell proliferation, increased cell death, and prevention of tumor initiation. Whereas androgen receptor (AR) positively regulates NKX3.1 expression, NKX3.1 negatively modulates AR transcription and consequently the AR-associated signaling events. Consistent with its tumor suppressor functions, NKX3.1 engages cell cycle and cell death machinery via association with HDAC1, leading to increased p53 acetylation and half-life through MDM2-dependent mechanisms. Importantly, overexpression of Nkx3.1 has little effect on Pten wild-type epithelium, suggesting that PTEN plays a predominant role in PTEN-NKX3.1 interplay. Manipulating NKX3.1 expression may serve as a therapeutic strategy for treating PTEN-deficient prostate cancers.3 In addition, NKX3.1 affects DNA damage response and cell survival after DNA damage. It was shown that endogenous NKX3.1 in LNCaP cells localizes to sites of DNA damage where it affects the recruitment of phosphorylated ATM and the phosphorylation of H2AX. NKX3.1 expression enhances activation of and activation of ATR.4 Moreover NKX3.1 activates expression of insulin-like growth factor binding protein-3 to attenuate insulin-like growth factor-I signaling and cell proliferation.5 thus, the growth-suppressive effects of NKX3.1 in prostate cells are mediated, in part, by activation of IGFBP-3 expression.
The Nkx3.1 homeobox gene is a key regulator of prostatic epithelial differentiation, while its loss-of-function in mice has been implicated in prostate cancer initiation. In particular, Nkx3.1 null mutant mice display abnormal prostatic differentiation as well as epithelial hyperplasia and dysplasia prior to 1 year of age. Human NKX3.1 maps to chromosome 8p21, which frequently undergoes loss of heterozygosity at early stages of prostate carcinogenesis. Although NKX3.1 is not mutated in prostate cancer, it undergoes epigenetic inactivation through loss of protein expression in human prostate cancer and in mouse models.2 It was shown that PTEN loss causes reduced NKX3.1 expression in both murine and human prostate cancers. Restoration of Nkx3.1 expression in vivo in Pten null epithelium leads to decreased cell proliferation, increased cell death, and prevention of tumor initiation. Whereas androgen receptor (AR) positively regulates NKX3.1 expression, NKX3.1 negatively modulates AR transcription and consequently the AR-associated signaling events. Consistent with its tumor suppressor functions, NKX3.1 engages cell cycle and cell death machinery via association with HDAC1, leading to increased p53 acetylation and half-life through MDM2-dependent mechanisms. Importantly, overexpression of Nkx3.1 has little effect on Pten wild-type epithelium, suggesting that PTEN plays a predominant role in PTEN-NKX3.1 interplay. Manipulating NKX3.1 expression may serve as a therapeutic strategy for treating PTEN-deficient prostate cancers.3 In addition, NKX3.1 affects DNA damage response and cell survival after DNA damage. It was shown that endogenous NKX3.1 in LNCaP cells localizes to sites of DNA damage where it affects the recruitment of phosphorylated ATM and the phosphorylation of H2AX. NKX3.1 expression enhances activation of and activation of ATR.4 Moreover NKX3.1 activates expression of insulin-like growth factor binding protein-3 to attenuate insulin-like growth factor-I signaling and cell proliferation.5 thus, the growth-suppressive effects of NKX3.1 in prostate cells are mediated, in part, by activation of IGFBP-3 expression.
REFERENCES
1. Shen, M.M. & Abate-Shen, C.: Dev. Dyn.228:767–78, 2003
2. Bora, G. et al: Am. J. Surg. Path. 34:1097-105, 2010
3. Lei, Q. et al: cancer Cell 9:367-78, 2006
4. Cai, B. & Gelmann, E.P: Cancer Res. 70:3089-97, 2010
5. Muhlbradt, E. et al: Cancer Res. 69:2615-22, 2009
2. Bora, G. et al: Am. J. Surg. Path. 34:1097-105, 2010
3. Lei, Q. et al: cancer Cell 9:367-78, 2006
4. Cai, B. & Gelmann, E.P: Cancer Res. 70:3089-97, 2010
5. Muhlbradt, E. et al: Cancer Res. 69:2615-22, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10341 |
Antigen: | Raised against recombinant human NKX3.1 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 - 1:200 ICC n/d FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 26 kDa |
Specificity/Sensitivity: | Detects endogenous NKX3.1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой