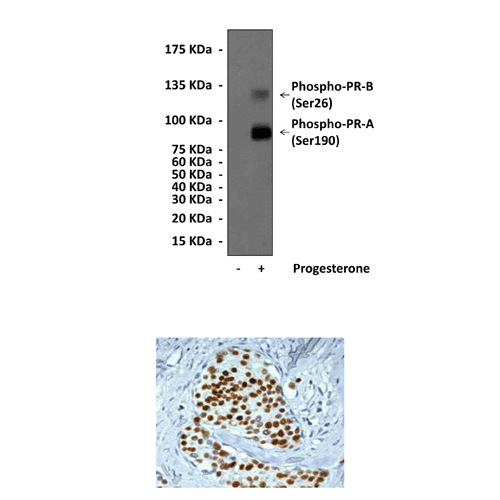
Anti-Phospho-PR: Rabbit Progesterone Receptor, Phospho-Ser190 Antibody |
 |
BACKGROUND The Progesterone Receptor (PR) also known as NR3C3 (Nuclear Receptor subfamily 3, group C, member 3), is an intracellular steroid receptor that specifically binds progesterone and mediates the physiologic effects of progesterone. The PR gene uses separate promoters and translational start sites to produce 2 isoforms, PR-A and PR-B, the only difference between the two being an additional 165 amino acids present in the N terminus of PR-B. In common with other steroid receptors, PR has a N-terminal regulatory domain, a DNA binding domain, a hinge section, and a C-terminal ligand binding domain. A special transcription activation function (TAF), called TAF-3, is present in the PR-B, in a B-upstream segment (BUS) at the amino acid terminal. This segment is not present in the receptor-A. Although human PR-B shares many important structural domains as human PR-A, they are in fact two functionally distinct transcription factors, mediating their own response genes and physiological effects with little overlap. The expression and ratios of PR-A and PR-B isoforms vary between normal and malignant tissues. In breast cancer cells with inducible PR-A or PR-B, progesterone stimulated gene regulation in a PR isoform-specific manner. The majority of the genes were uniquely regulated by PR-B, with smaller subsets regulated by PR-A or both isoforms. It has recently been demonstrated that PR gene regulation is further differentiated by whether or not the receptor is bound by the ligand. A majority of the genes regulated by the unliganded receptor were regulated by PR-A. The functions of PR isoforms in vivo recently have been characterized, based on studies in mice with selective ablation of PR-A and PR-B. Selective ablation of PR-A produced a phenotype characterized by infertility, severe endometrial hyperplasia, anovulation, and ovarian abnormalities in the presence of normal mammary gland response to progesterone. In contrast, mice with selective ablation of PR-B were fertile, did not show altered responses to progesterone in the uterus, but exhibited severely disrupted pregnancy-induced mammary gland morphogenesis.1
PR ligands bind to the PR, inducing dimerization and conformational changes that promote recruitment of coactivators or corepressors. The ligand-receptor complex, bound to the response element of the promoter region of the target genes, will induce or suppress transactivation of progesterone-target genes. The PR signaling may cross-talk with other cell signaling pathways. PR is a potent activator of Src kinases working by an SH3 domain displacement mechanism.2 It was shown that PR is phosphorylated on at least seven serine residues, which are all located in the amino-terminal (N) domain. The different kinetics of these phosphorylations in response to hormone suggests that these phosphorylation sites are targets of different signaling pathways and kinases and serve distinct functional/structural roles. At least three different kinases have been identified that specifically phosphorylate PR on authentic sites including casein kinase II on Ser 81, cyclin A-cyclin-dependent kinase 2 on Ser 162, Ser 190, and Ser 400, and MAP kinase on Ser 162 and Ser 294. Agents that either activate or inhibit cellular serine/threonine kinases and phosphatases in different signaling pathways have been observed to dramatically potentiate or inhibit the transcriptional activity of PR in cell transfection experiments.3 Additionally, activation of protein kinase A (PKA) through cAMP signaling leads to a functional switching of RU486 from an antagonist to a partial agonist on the B form of PR, but not through PR-A. Whether the influence of these agents on the transcriptional activity of PR is due to alterations of receptor phosphorylation directly, or to phosphorylation of other associated proteins involved in PR transactivation, appears to be dependent on the signaling pathway involved. In addition, PR is also a target of SUMO-1 modification.4
PR ligands bind to the PR, inducing dimerization and conformational changes that promote recruitment of coactivators or corepressors. The ligand-receptor complex, bound to the response element of the promoter region of the target genes, will induce or suppress transactivation of progesterone-target genes. The PR signaling may cross-talk with other cell signaling pathways. PR is a potent activator of Src kinases working by an SH3 domain displacement mechanism.2 It was shown that PR is phosphorylated on at least seven serine residues, which are all located in the amino-terminal (N) domain. The different kinetics of these phosphorylations in response to hormone suggests that these phosphorylation sites are targets of different signaling pathways and kinases and serve distinct functional/structural roles. At least three different kinases have been identified that specifically phosphorylate PR on authentic sites including casein kinase II on Ser 81, cyclin A-cyclin-dependent kinase 2 on Ser 162, Ser 190, and Ser 400, and MAP kinase on Ser 162 and Ser 294. Agents that either activate or inhibit cellular serine/threonine kinases and phosphatases in different signaling pathways have been observed to dramatically potentiate or inhibit the transcriptional activity of PR in cell transfection experiments.3 Additionally, activation of protein kinase A (PKA) through cAMP signaling leads to a functional switching of RU486 from an antagonist to a partial agonist on the B form of PR, but not through PR-A. Whether the influence of these agents on the transcriptional activity of PR is due to alterations of receptor phosphorylation directly, or to phosphorylation of other associated proteins involved in PR transactivation, appears to be dependent on the signaling pathway involved. In addition, PR is also a target of SUMO-1 modification.4
REFERENCES
1. Giangrande, P.H. & McDonnell, D.P.: Recent Prog. Horm. Res. 54:291-313, 1999
2. Boonyaratanakornkit, V. et al: Mol. Cell 8:269-80, 2001
3. Clemm, D.L. et al: Mol. Endocrinol. 14:52-65, 2000
4. Chauchereau, A. et al: J. Biol. Chem.. 278:12335-43, 2003
2. Boonyaratanakornkit, V. et al: Mol. Cell 8:269-80, 2001
3. Clemm, D.L. et al: Mol. Endocrinol. 14:52-65, 2000
4. Chauchereau, A. et al: J. Biol. Chem.. 278:12335-43, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CG1485
|
|
Antigen:
|
Short peptide from the human progesterone receptor sequence surrounding and containing phospho-Ser190.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Mouse, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000 - 1:10000
IP 1:50
IHC 1:100 - 1:250
ICC 1:100 - 1:250
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
90/118 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous phosphorylated PR-B (Ser190) and PR-A (Ser26) proteins without cross-reactivity with other family members.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой