Anti-PLGF: Rabbit Placenta Growth Factor Antibody |
 |
BACKGROUND Placenta growth factor (PLGF) is a close homolog of vascular endothelial growth factor (VEGF), shares receptors with VEGF, and stimulates angiogenesis. It is a pleiotropic cytokine that stimulates endothelial cell (EC) growth, migration, and survival; chemoattracts angiocompetent macrophages and bone marrow progenitors; and determines the metastatic niche. Unlike VEGF, PlGF together with VEGF-B belong to a subgroup of ligands within the VEGF family, which only binds to VEGFR1 and its coreceptors neuropilin-1 and -2. Consistent with the receptor binding patterns, PLGF and VEGF-B do not seem to display significant physiological functions.1 For example plgf or vegf-b-null mice develop normally and lack obvious vascular and non-vascular defects during the adulthood. However, recent studies show that PLGF might significantly contribute to pathological angiogenesis such as tumor neovascularization, vascular regeneration under tissue ischemia, and wound healing. Besides indirect effects, PlGF signals directly via VEGFR-1, thus, acting independently of VEGF in ECs, macrophages, bone marrow progenitors, and tumor cells, which primarily express VEGFR-1.2 In addition to its positive roles in regulation of pathological angiogenesis, PLGF has also been reported as a negative regulator of tumor angiogenesis and tumor growth. The mechanism of negative regulation of angiogenesis involves the formation of VEGF-PLGF heterodimers that do not display significantly angiogenic activity relative to VEGF homodimers. Four isoforms of human PLGF and six isoforms of human VEGF-A generated by alternative splicing from the same genes could potentially form 24 different heterodimers with different affinities to heparan sulfate proteoglycans, and thus create complex gradients around their producing cells.3 It was shown that PLGF levels in plasma and tumors correlate with tumor stage, vascularity, recurrence, metastasis, and survival in various tumors. Notably, PLGF is upregulated in cancer patients treated with VEGF(R) inhibitors therapy as well as in human tumors after radio-immunotherapy, suggesting a key role of PLGF in the angiogenic rescue.4 Genetic studies show that PLGF is redundant for vascular development and maintenance, but contributes to the angiogenic switch in disease. This raised the question whether PLGF inhibitors might reduce pathological angiogenesis but, unlike VEGF(R) inhibitors are, without affecting healthy blood vessels, and thus provide an attractive drug with a better safety profile.
REFERENCES
1. Ohno, S. & Nishizuka, Y.: J. Biochem. 132:509-11, 2002
2. Messerschmidt, A. et al: J. Mol. Biol. 352:918-31, 2005
3. Webb, B.L.J. et al: Br. J. Pharmcol. 130:1433-52, 2000
4. Parekh, D.B. et al: EMBO J. 19:496-503, 2000
2. Messerschmidt, A. et al: J. Mol. Biol. 352:918-31, 2005
3. Webb, B.L.J. et al: Br. J. Pharmcol. 130:1433-52, 2000
4. Parekh, D.B. et al: EMBO J. 19:496-503, 2000
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CA1066
|
|
Antigen:
|
Short peptide from human PLGF sequence.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000
IP n/d
IHC 1:50 - 1:200
ICC n/d
FACS n/d
|
|
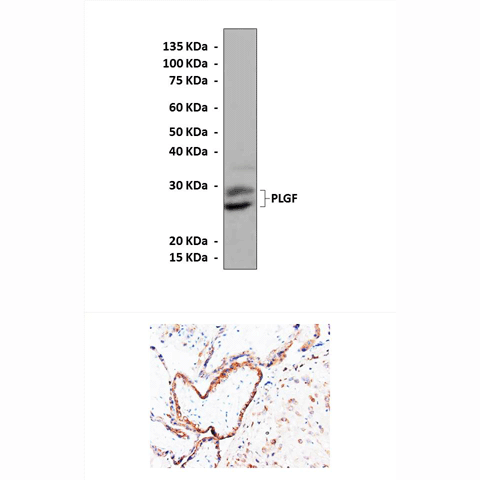
Predicted Molecular
Weight of protein:
|
26/28 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous levels of PLGF proteins without cross-reactivity with other related proteins.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой