Anti-Polyarginine: Rabbit Poly Arginine 9R Antibody |
 |
BACKGROUND Cell-penetrating peptides (CPPs) are small (10–30 residues in length), often positively charged sequences of amino acids, which were also termed ‘Trojan’ peptides. Most of them are water-soluble peptides with a low lytic activity and able to penetrate cell membranes and translocate different cargoes into cells independent of a membrane receptor and energy, show no cell-type specificity. CPPs have successfully improved the cellular uptake of various cargoes including proteins, nucleic acids (oligonucleotides, peptide-nucleic acids, siRNAs), nanoparticules and liposomes in a wide range of cells: mainly in mammalian cells, but also in bacteria, yeasts and protozoan parasites.1 The tat peptide, derived from HIV-1 transcriptional activator protein (tat), and penetratin, derived from Drosophila antennapedia (Antp) transcription protein, were the first cell-penetrating peptides (CPPs) to be described. During the last 15 years, more than 100 peptide sequences have been published to enter cells and also to bring different biological cargoes with them, which include many other naturally occurring CPPs such as the herpesvirus tegument protein VP22 or the cell wall protein-derived peptide inv3 from Mycobacterium tuberculosis, Chimaeric CPPs such as transportan (a chimera of the neuropeptide galanin and the wasp venom toxin mastoparan), and totally synthetic CPPs such as the model amphipathic peptide (MAP) or the polyarginine peptide etc. The exact mechanism of cellular uptake is not clear and studies remain controversial. Current models include uptake through transient pore formation, caveolae, clathrin dependent endocytosis, and macropinocytosis. Recent data suggest endocytosis as the prevailing model for uptake, although several mechanisms may coexist and differ depending on CPPs.2
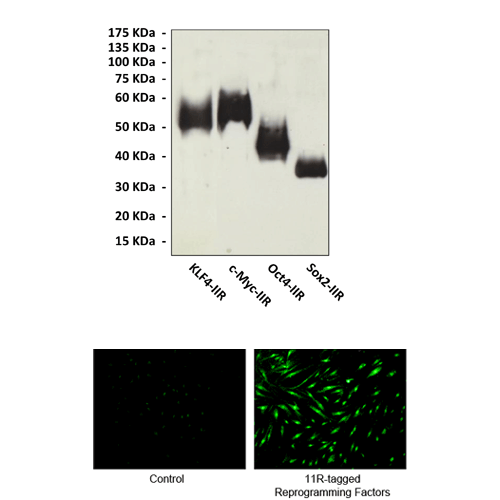
CPPs have proven to be very efficient in delivering molecules into cells that are refractory to transfection such as primary lymphocytes. Their penetration into cells is rapid and initially first-order, with half-times from 5 to 20 min. Recently, Dr. Sheng Ding’s lab designed and fused a poly-arginine (i.e., 11R) protein transduction domain to the C terminus of four reprogramming factors: Oct4, Sox2, Klf4, and c-Myc, and recombinant fusion proteins were made in E. coli. It was shown that the purified 11R-tagged recombinant transcription factors readily entered MEF cells which can be fully reprogrammed into pluripotent stem cells (iPSs) by direct delivery of recombinant reprogramming proteins.3 It is well known that the potential of iPS cells is enormous. However, the clinical application of iPS cells faces many obstacles. One of the challenges is that since the cells are artificially created to have pluripotency by inducing genes, they are inclined to form tumors. Dr. Ding’s nongenetic approach eliminates any risk of modifying the target cell genome by exogenous genetic sequences, which are associated with all previous iPSC methods. Thus, polyarginine-tagged protein transduction method provides a more efficient way of producing safer iPS cells without tendency to form tumors. In addition to acting as novel vehicle for the translocation of cargo into cells, polyarginie peptide has been used in various other biological applications. It was reported that oligoarginine chain was coupled with oligodeoxynucleotide probe to produce peptide-oligonucleotide conjugates or peptide-bridged oligonucleotide pairs. These probe-peptide conjugates induce the oligodeoxynucleotides to bind complementary single-stranded DNA or RNA targets with substantially enhanced thermal stability, which will be useful in the development of antisense strategies.4
CPPs have proven to be very efficient in delivering molecules into cells that are refractory to transfection such as primary lymphocytes. Their penetration into cells is rapid and initially first-order, with half-times from 5 to 20 min. Recently, Dr. Sheng Ding’s lab designed and fused a poly-arginine (i.e., 11R) protein transduction domain to the C terminus of four reprogramming factors: Oct4, Sox2, Klf4, and c-Myc, and recombinant fusion proteins were made in E. coli. It was shown that the purified 11R-tagged recombinant transcription factors readily entered MEF cells which can be fully reprogrammed into pluripotent stem cells (iPSs) by direct delivery of recombinant reprogramming proteins.3 It is well known that the potential of iPS cells is enormous. However, the clinical application of iPS cells faces many obstacles. One of the challenges is that since the cells are artificially created to have pluripotency by inducing genes, they are inclined to form tumors. Dr. Ding’s nongenetic approach eliminates any risk of modifying the target cell genome by exogenous genetic sequences, which are associated with all previous iPSC methods. Thus, polyarginine-tagged protein transduction method provides a more efficient way of producing safer iPS cells without tendency to form tumors. In addition to acting as novel vehicle for the translocation of cargo into cells, polyarginie peptide has been used in various other biological applications. It was reported that oligoarginine chain was coupled with oligodeoxynucleotide probe to produce peptide-oligonucleotide conjugates or peptide-bridged oligonucleotide pairs. These probe-peptide conjugates induce the oligodeoxynucleotides to bind complementary single-stranded DNA or RNA targets with substantially enhanced thermal stability, which will be useful in the development of antisense strategies.4
REFERENCES
1. Morris, M.C. et al: Biol. Cell 100:201–17, 2008
2. Sawant, R. & Torchilin, V.: Mol. BioSyst., 6:628-640, 2010
3. Zhou, H. et al: Cell Stem Cell 4:381-4, 2009
4. Wei, Z. et al: Nucl. Acids Res. 24:655-61, 1996
2. Sawant, R. & Torchilin, V.: Mol. BioSyst., 6:628-640, 2010
3. Zhou, H. et al: Cell Stem Cell 4:381-4, 2009
4. Wei, Z. et al: Nucl. Acids Res. 24:655-61, 1996
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CY1114 |
Antigen: | Short 9-mer arginine peptide coupled with KLH. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Arginine-tag |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 1 kDa + Target Protein |
Specificity/Sensitivity: | Detects 9R-tagged proteins without cross-reactivity with un-tagged proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой