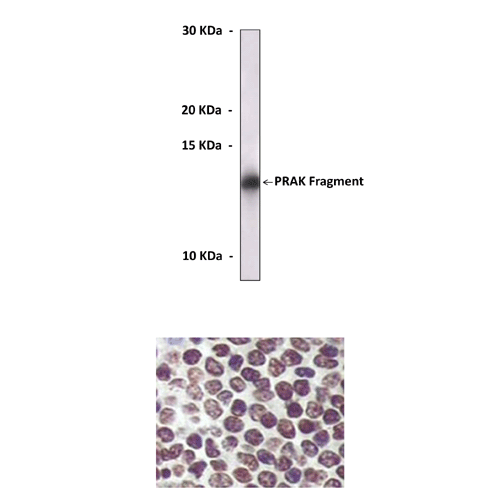
Anti-PRAK: Mouse PRAK/MK5 Antibody |
 |
BACKGROUND p38 MAPK mediates multiple cellular processes, including proliferation, differentiation, inflammation, and stress responses. p38-regulated/activated protein kinase (PRAK, or MK5) is one member of the p38 downstream Ser/Thr protein kinase family, which also includes MK2, MK3, MNK1, MNK2, and MSK. PRAK can be activated in response to cellular stress and proinflammatory cytokines. PRAK activity was regulated by p38alpha and p38beta in vitro and in vivo through phosphorylation. Thr182 within the activation loop of PRAK has been determined to be the regulatory phosphorylation site. PRAK was also activated by atypical MAPK ERK3 and 4.1
PRAK was first identified to be involved in regulation of cytoskeleton remodeling and embryo development. It was also shown that PRAK−/− mice display normal LPS-induced cytokine production and endotoxic shock, suggesting that PRAK may not be essential for inflammation and stress responses. The physiological role and downstream target of PRAK thus remain undefined. It was shown that small heat shock protein 27 (Hsp27) and the regulatory light chain of myosin II are the potential substrates of PRAK. PRAK may play a role in balancing other MAPK pathways because overactivation of PRAK can inhibit Ras mediated cell proliferation and gene activation. It was uncovered recently that PRAK has a novel function in tumor suppression and ras-induced senescence. PRAK deficiency leads to increased skin carcinogenesis in mice and renders primary cells more susceptible to oncogenic transformation. The tumor-suppressing effect of PRAK is likely due to its ability to mediate ras-induced senescence, by modulating p53 activity through direct phosphorylation. There is a signaling cascade that plays an essential role in ras-induced senescence and tumor suppression.2 PRAK may participate in cell cycle regulation. PRAK gene knockout in MEF cells leads to cell cycle arrest and proliferation inhibition.3 Current available data of PRAK show almost no difference between PRAK and MK2 in their activation profile and substrate specificity. However, PRAK should have different functions in comparison with MK2 because it cannot compensate for MK2 deficiency in cells. Because of the importance of subcellular location in protein function, it was shown that PRAK is localized predominantly in the cytoplasm and is constantly shuttled between the cytoplasm and the nucleus.4
PRAK was first identified to be involved in regulation of cytoskeleton remodeling and embryo development. It was also shown that PRAK−/− mice display normal LPS-induced cytokine production and endotoxic shock, suggesting that PRAK may not be essential for inflammation and stress responses. The physiological role and downstream target of PRAK thus remain undefined. It was shown that small heat shock protein 27 (Hsp27) and the regulatory light chain of myosin II are the potential substrates of PRAK. PRAK may play a role in balancing other MAPK pathways because overactivation of PRAK can inhibit Ras mediated cell proliferation and gene activation. It was uncovered recently that PRAK has a novel function in tumor suppression and ras-induced senescence. PRAK deficiency leads to increased skin carcinogenesis in mice and renders primary cells more susceptible to oncogenic transformation. The tumor-suppressing effect of PRAK is likely due to its ability to mediate ras-induced senescence, by modulating p53 activity through direct phosphorylation. There is a signaling cascade that plays an essential role in ras-induced senescence and tumor suppression.2 PRAK may participate in cell cycle regulation. PRAK gene knockout in MEF cells leads to cell cycle arrest and proliferation inhibition.3 Current available data of PRAK show almost no difference between PRAK and MK2 in their activation profile and substrate specificity. However, PRAK should have different functions in comparison with MK2 because it cannot compensate for MK2 deficiency in cells. Because of the importance of subcellular location in protein function, it was shown that PRAK is localized predominantly in the cytoplasm and is constantly shuttled between the cytoplasm and the nucleus.4
REFERENCES
1. Aberg, E. et al: J. Biol. Chem. 284:19392-401, 2009
2. Sun, P. et al: Cell 128:295-308, 2007
3. Gong, X. et al: Front. Med. China 3:379-83, 2009
4. New, L. et al: Mol. Biol. Cell 14:2603-16, 2003
2. Sun, P. et al: Cell 128:295-308, 2007
3. Gong, X. et al: Front. Med. China 3:379-83, 2009
4. New, L. et al: Mol. Biol. Cell 14:2603-16, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10204 |
Antigen: | Purified recombinant human PRAK fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 54 kDa |
Specificity/Sensitivity: | Detects PRAK proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
|
| ||||||||||
| ||||||||||
Extended Family Products
|
| ||||||||||
| ||||||||||
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой