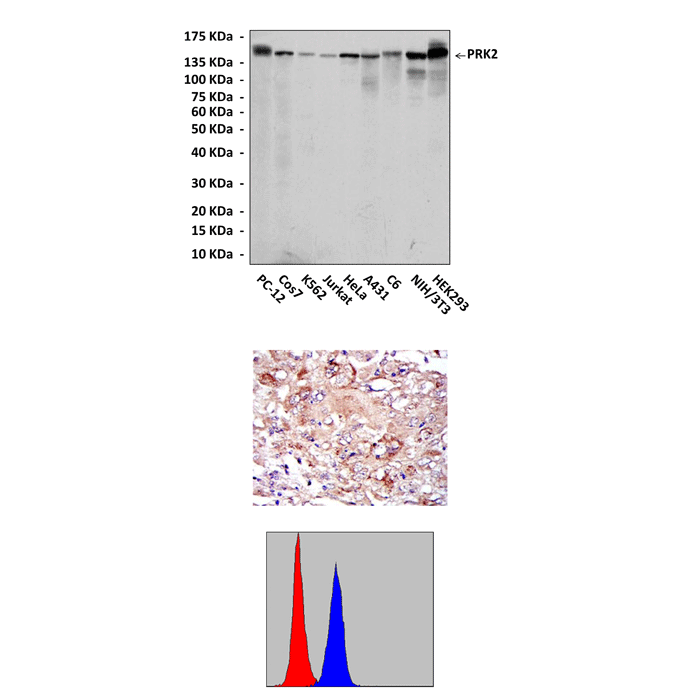
Anti-PRK2/PKN: PRK2/PKN Antibody |
 |
BACKGROUND PKNs (Protein Kinase Novel, also known as PRKs) are a subfamily of PKC related AGC serine/threonine kinases. The carboxyl-terminal kinase domains of these proteins are closely related to those of PKC, and at their amino termini they have three repeats of a leucine-zipper-like motif (HR1a,b,c) followed by a C2-related domain; overall these proteins have a domain organization related to that of the yeast PKC-related proteins. The C-terminal half of PRK2 contains various motifs which involved in its kinase activity and regulation. Two threonine residues at conserved phosphoacceptor position in the activation loop and the turn motif are essential for the catalytic activity of PRK2. Both the intact hydrophobic motif and the turn motif in PRK2 are dispensable for the binding of PDK-1.1 In addition, PKNs are activated by fatty acids, limited proteolysis, and phospholipids. Moreover, PKNs are direct Rho effectors. PRK2 was activated by both Rho and Rac.2 It was shown that both PKN1/PRK1 and PRK2 bind to Rho via an N-terminal Rho effector homology (REM) region which contains HR1 motifs. Moreover, the extreme C-terminal segment is critical for the full activation of PRK2 by RhoA in cells in a GTP-dependent manner. Rho/PRK2 signaling is involved in regulation of cell skeletons. It was demonstrated that Fyn is a downstream mediator of Rho in control of keratinocyte cell–cell adhesion and implicate the PRK2 kinase as a link between Rho and Fyn activation.3 In addition other signaling components may be involved in Rho/PRK2/ cell skeleton remodeling regulation. PKR2 was found to interact with protein tyrosine phosphatase-basophil like (PTP-BL) in lamellipodia like structures in HeLa cells. PTP-BL a large non-transmembrane protein tyrosine phosphatase implicated in the modulation of the cytoskeleton. PTP-BL might function as a scaffold for proteins involved in rho/rac signal transduction and thereby regulating the cytoskeleton.4
There is accumulating evidence that some of the functions of PRK2 are independent of the cytoskeletal effects of Rho. It was reported that PRK2 may regulate translation initiation during oocyte maturation by phosphorylating the serine-209 residue of eIF4E in starfish. Moreover, high levels of cAMP inhibit the activation of PRK2, eIF4E, and the eIF4E binding protein during starfish oocyte maturation, while PI3 kinase activates these proteins.5 Moreover, PRK2 is a candidate regulator of early signaling events of meiotic maturation.
There is accumulating evidence that some of the functions of PRK2 are independent of the cytoskeletal effects of Rho. It was reported that PRK2 may regulate translation initiation during oocyte maturation by phosphorylating the serine-209 residue of eIF4E in starfish. Moreover, high levels of cAMP inhibit the activation of PRK2, eIF4E, and the eIF4E binding protein during starfish oocyte maturation, while PI3 kinase activates these proteins.5 Moreover, PRK2 is a candidate regulator of early signaling events of meiotic maturation.
REFERENCES
1. Torbett, N.E. et al: J. Biol. Chem. 278:32344-51, 2003
2. Vincent, S. & Settleman, J.: Mol. Cell. Biol. 17:2247-56, 1997
3. Calautti, E. et al: J. Cell Biol. 156:137-48, 2002
4. Gross, C. et al: FEBS Lett. 496:101-4, 2001
5. Lee, S-J. et al: Dev. Biol. 228: 166-180, 2000
2. Vincent, S. & Settleman, J.: Mol. Cell. Biol. 17:2247-56, 1997
3. Calautti, E. et al: J. Cell Biol. 156:137-48, 2002
4. Gross, C. et al: FEBS Lett. 496:101-4, 2001
5. Lee, S-J. et al: Dev. Biol. 228: 166-180, 2000
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10412 |
Antigen: | Raised against recombinant human PRK2 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 140 kDa |
Specificity/Sensitivity: | Detects PRK2 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой