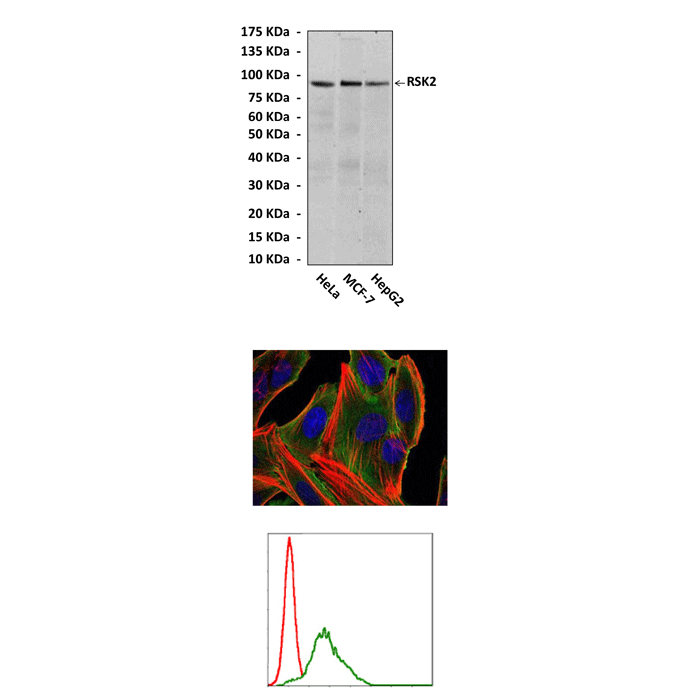
Anti-RSK2: Mouse RSK2 Antibody Product |
 |
BACKGROUND The 90 kDa Ribosomal S6 Kinases (RSK) are a family of broadly expressed serine/threonine kinases that are activated by Extracellular Signal-Regulated Protein Kinase (ERK1 and ERK2) in response to many growth factors, peptide hormones and neurotransmitters. In humans, the RSK family consists of four isoforms (RSK1 to -4) and two structurally related cousins, called RSK-like protein kinase (RLPK/MSK1) and RSK-B (MSK2).1 RSK family members are unusual among serine/threonine kinases in that they contain two distinct kinase domains, both of which are catalytically functional. The C-terminal kinase domain is believed to be involved in autophosphorylation, a critical step in RSK activation, whereas the N-terminal kinase domain, which is homologous to members of the AGC superfamily of kinases, is responsible for the phosphorylation of all known exogenous substrates of RSK. Activated RSK has both cytoplasmic and nuclear substrates. RSK plays an active role in nuclear signaling by phosphorylating the transcription factors like estrogen receptor-alpha and CREB, and c-Fos, and IkappaB. RSK has been proposed to bind to and modulate the function of CBP and p300, which are transcriptional co-activators. Phosphorylation of Bad and C/EBPbeta by RSK can protect cells from apoptosis. RSK has also been implicated in cell cycle regulation.2 In Xenopus extracts, RSK phosphorylates and inhibits Myt1, a p34cdc2 inhibitory kinase. Moreover, RSK phosphorylates histone H3, suggesting that RSK may regulate chromatin remodeling.3
The precise mechanism of RSK activation remains elusive. The current model suggests that following mitogen stimulation, ERK phosphorylates RSK1 Thr590 (according to avian RSK1 numbering; human 573), located in the activation loop of the C-terminal kinase domain, and possibly Ser381 (turn motif; human 363) and Thr377 (human 359) in the linker region between the two kinase domains. Activation of RSK1 is absolutely dependent on ERK docking near the C terminus of RSK1. Activation of the C-terminal kinase domain leads to autophosphorylation of Ser398 (hydrophobic motif; human 380), also located in the linker region. This creates a docking site for PDK1, which then phosphorylates Ser239 (human 221) in the activation loop of the N-terminal kinase domain, allowing RSK to phosphorylate all its targets.4 Interestingly, Ser749 (human 732) is located near the ERK docking site and represents another mitogen-regulated phosphorylation site in RSK1. This residue is thought to be regulated by autophosphorylation from the N-terminal kinase domain, as it fits a RSK consensus phosphorylation sequence (K/RXXS/T). Ser749 phosphorylation regulates interaction between ERK1/2 and RSK1. Moreover, mutations that affect RSK1 kinase activity prevent ERK1/2 dissociation from RSK1 following mitogen stimulation in vivo. Analysis of different RSK isoforms revealed that RSK1 and RSK2 dissociate from ERK1/2 following mitogen stimulation but that RSK3 remains associated with active ERK1/2. ERK dissociation from RSK1 and RSK2 correlated with shorter duration of activity compared to RSK3, indicating that ERK dissociation may shorten RSK activation.5
The precise mechanism of RSK activation remains elusive. The current model suggests that following mitogen stimulation, ERK phosphorylates RSK1 Thr590 (according to avian RSK1 numbering; human 573), located in the activation loop of the C-terminal kinase domain, and possibly Ser381 (turn motif; human 363) and Thr377 (human 359) in the linker region between the two kinase domains. Activation of RSK1 is absolutely dependent on ERK docking near the C terminus of RSK1. Activation of the C-terminal kinase domain leads to autophosphorylation of Ser398 (hydrophobic motif; human 380), also located in the linker region. This creates a docking site for PDK1, which then phosphorylates Ser239 (human 221) in the activation loop of the N-terminal kinase domain, allowing RSK to phosphorylate all its targets.4 Interestingly, Ser749 (human 732) is located near the ERK docking site and represents another mitogen-regulated phosphorylation site in RSK1. This residue is thought to be regulated by autophosphorylation from the N-terminal kinase domain, as it fits a RSK consensus phosphorylation sequence (K/RXXS/T). Ser749 phosphorylation regulates interaction between ERK1/2 and RSK1. Moreover, mutations that affect RSK1 kinase activity prevent ERK1/2 dissociation from RSK1 following mitogen stimulation in vivo. Analysis of different RSK isoforms revealed that RSK1 and RSK2 dissociate from ERK1/2 following mitogen stimulation but that RSK3 remains associated with active ERK1/2. ERK dissociation from RSK1 and RSK2 correlated with shorter duration of activity compared to RSK3, indicating that ERK dissociation may shorten RSK activation.5
REFERENCES
1. Lee, K.Y. et al: Signal Transduct. 7:225-39, 2007
2. Anjum, R. & Blenis, J.: Nature Rev. Mol. Cell. Biol. 9:747-58, 2008
3. Frödin, M. & Gammeltoft, S.:Mol. Cell. Endocrin. 151:65-77, 1999
4. Jensen, C.J. et al: J. Biol. Chem. 274:27168-74, 1999
5. Roux, P.P. et al: Mol. Cell. Biol. 23:4796-804, 2003
2. Anjum, R. & Blenis, J.: Nature Rev. Mol. Cell. Biol. 9:747-58, 2008
3. Frödin, M. & Gammeltoft, S.:Mol. Cell. Endocrin. 151:65-77, 1999
4. Jensen, C.J. et al: J. Biol. Chem. 274:27168-74, 1999
5. Roux, P.P. et al: Mol. Cell. Biol. 23:4796-804, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10433 |
Antigen: | Raised against recombinant human RSK2 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 - 1:100 IHC n/d ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 90 kDa |
Specificity/Sensitivity: | Detects RSK2 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой