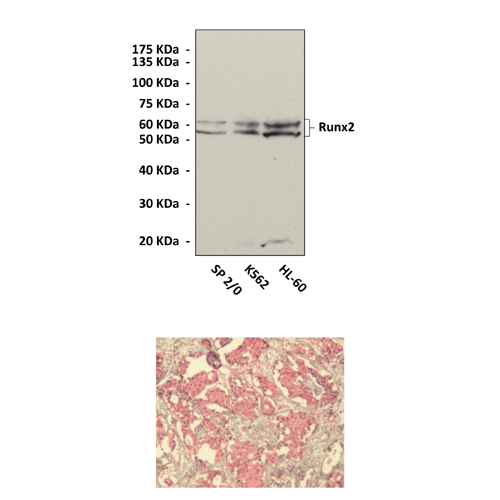
Anti-Runx2/CBFA1: Rabbit Runx2/CBFA1 Antibody |
 |
BACKGROUND The transcription factor Runx2, also called CBFA1/OSF2/AML3/PEBP2αA, is one of the three mammalian members of the Runt-related transcription family. Runx2 is characterized by a highly conserved runt homology DNA-binding domain (RHD) in the N-terminus, and the C-terminus contains a nuclear matrix-associated regulatory domain (nuclear matrix-targeting signal—NMTS) that targets Runx2 to subnuclear foci. Runx2 is a master transcription factor of bone and plays a role in all stages of bone formation. It is essential for the initial commitment of mesenchymal cells to the osteoblastic lineage and also controls the proliferation, differentiation, and maintenance of these cells. Control is complex, with involvement of a multitude of factors, thereby regulating the expression and activity of this gene both temporally and spatially. RUNX2 is also essential for the later stages of tooth formation, is intimately involved in the development of calcified tooth tissue, and exerts an influence on proliferation of the dental lamina. Furthermore, RUNX2 regulates the alveolar remodeling process essential for tooth eruption and may play a role in the maintenance of the periodontal ligament.1 Mutations in Runx2 gene have been associated with the bone development disorder cleidocranial dysplasia (CCD), which is characterized by clavicular hypoplasia and craniofacial abnormalities owing to the maturational arrest of osteoblasts caused by Runx2 mutations. Moreover, persons with Runx2 gene mutations also display dental disorders, with supernumerary teeth, abnormal tooth eruption, and tooth hypoplasia. Runx2 null mice exhibit a complete lack of both intramembranous and endochondral ossification due to an absence of osteoblasts and consequently an unmineralized skeleton.2 Several lines of evidence indicate that Runx2 also plays an important role in adipogenesis and skeletal muscle differentiation. The accumulation of lipid droplets and increased adipogenic gene expression are observed in chondrocyte cells derived from Runx2 null mice.3 Runx2 association with the nuclear scaffold facilitates interaction with many co-regulatory proteins and chromatin-modifying complexes for the regulation of gene transcription. The Runx2 coactivator TAZ and CCAAT/enhancer binding protein beta (C/EBP-beta) promote osteogenesis, whereas they inhibit adipogenesis, in mesenchymal cells. Runx2 prevents myogenesis and myotube formation via the suppression of MyoD and myogenin transcripts.4 Thus, Runx2 broadly modulates cellular fate, including cell traits. Defining the molecular mechanisms by which Runx2 can function as a master regulatory gene for activating the program of osteoblastogenesis has provided novel insights for transcriptional regulation of tissue-specific genes. Regulation of Runx expression has the potential to serve as a basis for the design of novel therapeutic strategies for promoting bone formation. In addition, use of multiple promoters and alternative splicing of exons generate various Runx2 isoforms and further extends its diversity of actions.5
REFERENCES
1. Lian, J.B. & Stein, G.S.: Curr. Pharm. Des. 9:2677-85, 2003
2. Ducy, P.: Dev Dyn. 219:461-71, 2000
3. Karsenty, G.: Semin. Cell. Dev. Biol. 11:343-6, 2000
4. Tanaka, T. et al: Mol. Cell. Biol. 28:1147-60, 2008
5. Banerjee, C. et al: Endocrinol. 142:4026-39, 2001
2. Ducy, P.: Dev Dyn. 219:461-71, 2000
3. Karsenty, G.: Semin. Cell. Dev. Biol. 11:343-6, 2000
4. Tanaka, T. et al: Mol. Cell. Biol. 28:1147-60, 2008
5. Banerjee, C. et al: Endocrinol. 142:4026-39, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CY1111 |
Antigen: | Short peptide from C-terminal sequence of human Runx2 protein. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 52/62 kDa |
Specificity/Sensitivity: | Detects endogenous Runx2 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой