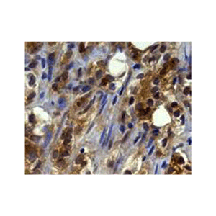
Anti-Smurf2: Rabbit Smurf2 (IHC/ICC-specific) Monoclonal Antibody |
 |
BACKGROUND In general, three enzymes catalyze the ubiquitination reaction: a Ub-activating enzyme (E1), a Ub-conjugating enzyme (E2), and a specificity-determining Ub-protein ligase (E3). Ub ligases fall into two categories; HECT domain E3s form a Ub thioester intermediate via a strictly conserved cysteine residue, while RING finger and U-box E3s act as scaffolding proteins that indirectly enhance Ub transfer from the E2 conjugate to the substrate. One class of HECT domain E3s is characterized by a common modular organization with an N-terminal C2 domain that can mediate membrane localization, two to four WW domains that recognize proline-rich motifs of substrates or adaptor proteins, and a C-terminal HECT domain that catalyzes isopeptide bond formation between the Ub C terminus and the substrate lysine residue.1
Smurf1 (Smad ubiquitination-related factor 1) and Smurf2 are closely related C2-WW-HECT-domain E3s that play important roles in the downregulation of the TGF-beta signaling pathway.2 TGF-beta proteins are key morphogens that control a variety of developmental processes, including cell growth, differentiation, and self-renewal. Interaction of TGF-beta morphogens with TGF-beta receptor complexes initiates intracellular signaling cascades involving Smad proteins and the polarity protein Par6. Upon TGF-beta-induced expression, the inhibitory adaptor protein Smad7 forms a complex with the Smurf2 Ub ligase in the nucleus. Association of Smurf2 with Smad7 serves at least three regulatory functions; it stimulates Smurf activity by recruiting the E2 to the HECT domain, induces the export of the Smurf2-Smad7 complex to the cytoplasm, and mediates its interaction with TGF-beta receptors resulting in the ubiquitination of TGF-beta receptors, Smad7, and Smurf2 itself to ultimately terminate TGF-beta signaling.3
Smurf proteins also regulate cell shape, motility, and polarity by degrading small guanosine triphosphatases (GTPases). While Smurf2-dependent degradation of the GTPase Rap1B is required for the establishment of neuronal polarity, Smurf1 targets RhoA in migrating and polarized epithelial cells. Smurf1 activity toward RhoA is spatially restricted to membrane protrusions in migrating cells or tight junction regions in epithelial cells by the polarity proteins atypical protein kinase C and Par6. Growing evidence suggests that dysregulation of Smurf-dependent degradation of components of TGF-beta signaling pathways leads to developmental defects and plays important roles in cancer pathogenesis and metastasis.4 Additionally it was demonstrated that the ubiquitination machinery itself is also regulated to prevent autocatalytic degradation of the core components. The HECT-type E3s are subject to regulation either by E3 or substrate phosphorylation or by utilization of adaptor proteins that facilitate E2 recruitment. It was also found that an intramolecular interaction between the C2 and HECT domains inhibits Smurf2 activity, stabilizes Smurf2 levels in cells, and similarly inhibits certain other C2-WW-HECT-domain E3s. The HECT-binding domain of Smad7, which activates Smurf2, antagonizes this inhibitory interaction. Thus, interactions between C2 and HECT domains autoinhibit a subset of HECT-type E3s to protect them and their substrates from futile degradation in cells.5
Smurf1 (Smad ubiquitination-related factor 1) and Smurf2 are closely related C2-WW-HECT-domain E3s that play important roles in the downregulation of the TGF-beta signaling pathway.2 TGF-beta proteins are key morphogens that control a variety of developmental processes, including cell growth, differentiation, and self-renewal. Interaction of TGF-beta morphogens with TGF-beta receptor complexes initiates intracellular signaling cascades involving Smad proteins and the polarity protein Par6. Upon TGF-beta-induced expression, the inhibitory adaptor protein Smad7 forms a complex with the Smurf2 Ub ligase in the nucleus. Association of Smurf2 with Smad7 serves at least three regulatory functions; it stimulates Smurf activity by recruiting the E2 to the HECT domain, induces the export of the Smurf2-Smad7 complex to the cytoplasm, and mediates its interaction with TGF-beta receptors resulting in the ubiquitination of TGF-beta receptors, Smad7, and Smurf2 itself to ultimately terminate TGF-beta signaling.3
Smurf proteins also regulate cell shape, motility, and polarity by degrading small guanosine triphosphatases (GTPases). While Smurf2-dependent degradation of the GTPase Rap1B is required for the establishment of neuronal polarity, Smurf1 targets RhoA in migrating and polarized epithelial cells. Smurf1 activity toward RhoA is spatially restricted to membrane protrusions in migrating cells or tight junction regions in epithelial cells by the polarity proteins atypical protein kinase C and Par6. Growing evidence suggests that dysregulation of Smurf-dependent degradation of components of TGF-beta signaling pathways leads to developmental defects and plays important roles in cancer pathogenesis and metastasis.4 Additionally it was demonstrated that the ubiquitination machinery itself is also regulated to prevent autocatalytic degradation of the core components. The HECT-type E3s are subject to regulation either by E3 or substrate phosphorylation or by utilization of adaptor proteins that facilitate E2 recruitment. It was also found that an intramolecular interaction between the C2 and HECT domains inhibits Smurf2 activity, stabilizes Smurf2 levels in cells, and similarly inhibits certain other C2-WW-HECT-domain E3s. The HECT-binding domain of Smad7, which activates Smurf2, antagonizes this inhibitory interaction. Thus, interactions between C2 and HECT domains autoinhibit a subset of HECT-type E3s to protect them and their substrates from futile degradation in cells.5
REFERENCES
1. Ingham, R.J. et al: Oncogene 23:1972-84, 2004
2. Zhu, H. et al: Nature 400:687-93, 1999
3. Kavsak, P. et al: Mol. Cell 6:1365-75, 2000
4. Izzi L. & Attisano, L.:Oncoegen 23:2071-8, 2004
5. Wiesner, S. et al: Cell 130:651-62, 2007
2. Zhu, H. et al: Nature 400:687-93, 1999
3. Kavsak, P. et al: Mol. Cell 6:1365-75, 2000
4. Izzi L. & Attisano, L.:Oncoegen 23:2071-8, 2004
5. Wiesner, S. et al: Cell 130:651-62, 2007
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CG1734
|
|
Antigen:
|
Short peptide from human Smurf2 sequence.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:150
IP n/d
IHC 1:100 - 1:250
ICC 1:50
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
86 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous Smurf2 proteins in IHC/ICC assays without cross-reactivity with other family members.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Extended Family Products
|
| ||||||||||
| ||||||||||
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой