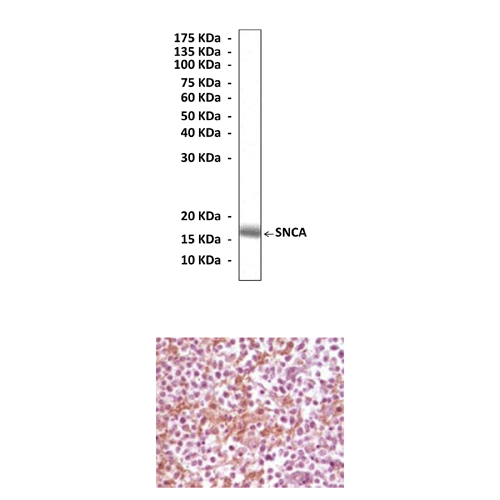
Anti-SNCA: Mouse Alpha-Synuclein Antibody |
 |
BACKGROUND Alpha-synuclein (α-Syn, SNCA) is a short 140-amino acid neuronal protein localized in the presynaptic compartment of neurons that has been found to be expressed throughout the brain. SNCA appears to play a role in regulating the distal pool of presynaptic vesicles and dopamine release or functions as a chaperone. It has been shown that SNCA regulates the homeostasis of monoamine neurotransmitters, through its trafficking, and regulation of the cell surface expression and, thereby, the activity of dopamine, serotonin and norepinephrine transporters. Furthermore, the N-terminus of SNCA contains several imperfect KTKEGV repeats, which are thought to mediate binding to membranes by interacting with phospholipids and shifting from a random coil to an -helical conformation. The central region of SNCA is known as the NAC domain, and it contains hydrophobic amino acids required for fibrillization, particularly residues 71-82. SNCA is involved in various degenerative disorders such as Parkinson's disease, dementia with Lewy bodies, which are recognized as alpha-synucleinopathies. Mutations in the SNCA gene are causal for familial Parkinson disease/Lewy body disease. Interestingly, the PD familial mutation A30P in SNCA showed reduced binding to membranes due to the Ala Prosubstitution in repeat 2, which disrupts membrane interaction. It is clear that point mutations or increased expression of wild type α-synuclein causes disease. A great deal of literature supports the overall hypothesis that α-synuclein is damaging to neurons because it is inherently prone to aggregation; mutations or increased concentration of the protein both increase this tendency. Moreover, α-Synuclein gene (SNCA) multiplication was found in familial and sporadic Parkinson disease (PD).1 It was demonstrated that a copy number variation of the SNCA gene is associated with selective impairments on reinforcement learning in asymptomatic carriers without the motor symptoms of Parkinson disease.2 It is suggested that the toxic species are small oligomers that are relatively soluble, which may react with membranes to damage key processes within the cell. Derangements in vesicle processing, including synaptic function, protein turnover, mitochondrial function and oxidative stress, have all been suggested to occur. The outcome is to trigger cell death, by both apoptotic and non-apoptotic mechanisms depending on the system studied.3 Furthermore, It was shown that α-Syn may also play a pathophysiological role in depressive symptoms.
REFERENCES
1. Ahn, T.B. et al: Neurol. 70:43-9, 2008
2. Keri, S. et al: Proc. Natl. Acad. Sci. USA 107:15992-4, 2010
3. Cookson, M.R. & van der Brug, M.: Exp. Neurol. 209:5-11, 2008
2. Keri, S. et al: Proc. Natl. Acad. Sci. USA 107:15992-4, 2010
3. Cookson, M.R. & van der Brug, M.: Exp. Neurol. 209:5-11, 2008
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10383 |
Antigen: | Raised against purified recombinant fragments of human SNCA expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 16 kDa |
Specificity/Sensitivity: | Detects SNCA proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой