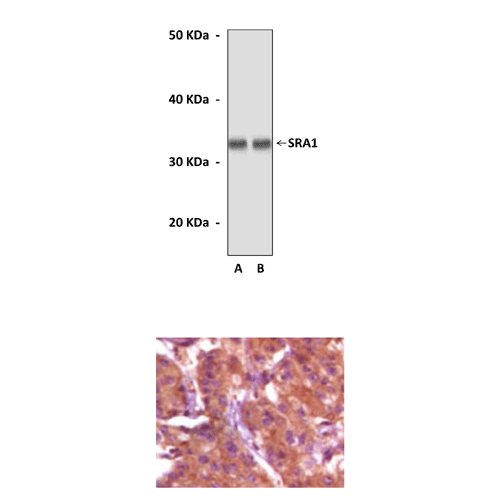
Anti-SRA1: Mouse Steroid Receptor RNA Activator Antibody |
 |
BACKGROUND Steroid receptor RNA activator (SRA) acts as an RNA transcript by regulating eukaryotic gene expression mediated by the steroid receptors (SRs), which play critical roles in eukaryotic development, metabolism, reproduction, and disease. It was demonstrated that SRA enhances cellular proliferation and differentiation and promotes apoptosis in vivo.1 It may also play a role in tumorigenesis. It was shown that changes in the expression of SRA-related molecules occur during breast tumor progression.2
In mammalian tissue culture cells, recombinant SRA showed potent coactivation activity with the receptors for androgens (AR), estrogens (ER), glucocorticoids, and progestins (PR). However, no evidence has yet been obtained for a direct binding of SRA to SRs. It was found that SRA is in a protein complex together with SRC coactivators and therefore suggested that SRA-containing ribonucleoprotein complexes accentuate transcriptional specificity by integrating coregulator activities upon selective binding to SRs. Endorsing this model, SRA was shown to associate with the DEAD box proteins p72/p68 to act as an ERalpha-specific ribonucleoprotein coactivator complex, which stimulates the amino-terminal activation domain of the receptor while concurrently integrating SRC/p160-mediated AF2 coactivator functions. Similarly, SRA was shown to bind to SHARP to modulate ER transactivation by attenuating the steroid response through sequestration while simultaneously initiating repression by SMRT. An analogous mechanism of transcriptional control has also been suggested for RTA (for "repressor of tamoxifen transcriptional activity"), a negative coregulator for ER.1 Additionally it was shown that a set of discrete stem-loop structures within SRA are required for its coactivation function.
Recently, it was reported that the steroid receptor RNA activator gene (SRA1) encodes for a functional RNA (SRA) as well as a protein (SRAP). SRA1 is a SRA isoform. SRAP was found to enhance estrogen receptor alpha activity in a ligand and response-element dependent manner. Thus, both transcript and protein products of the SRA1 gene co-modulate the transcriptional activity of steroid receptors.3 SRAP physically interacts with multiple transcription factors and is recruited to specific promoter regions. Artificially recruiting SRAP to the promoter of a luciferase reporter gene under the control of the strong transcriptional activator VP16 leads to a decrease in transcription. It is suggested that that SRAP could be a new transcriptional regulator, able to function as a repressor through direct association with promoters.4
In mammalian tissue culture cells, recombinant SRA showed potent coactivation activity with the receptors for androgens (AR), estrogens (ER), glucocorticoids, and progestins (PR). However, no evidence has yet been obtained for a direct binding of SRA to SRs. It was found that SRA is in a protein complex together with SRC coactivators and therefore suggested that SRA-containing ribonucleoprotein complexes accentuate transcriptional specificity by integrating coregulator activities upon selective binding to SRs. Endorsing this model, SRA was shown to associate with the DEAD box proteins p72/p68 to act as an ERalpha-specific ribonucleoprotein coactivator complex, which stimulates the amino-terminal activation domain of the receptor while concurrently integrating SRC/p160-mediated AF2 coactivator functions. Similarly, SRA was shown to bind to SHARP to modulate ER transactivation by attenuating the steroid response through sequestration while simultaneously initiating repression by SMRT. An analogous mechanism of transcriptional control has also been suggested for RTA (for "repressor of tamoxifen transcriptional activity"), a negative coregulator for ER.1 Additionally it was shown that a set of discrete stem-loop structures within SRA are required for its coactivation function.
Recently, it was reported that the steroid receptor RNA activator gene (SRA1) encodes for a functional RNA (SRA) as well as a protein (SRAP). SRA1 is a SRA isoform. SRAP was found to enhance estrogen receptor alpha activity in a ligand and response-element dependent manner. Thus, both transcript and protein products of the SRA1 gene co-modulate the transcriptional activity of steroid receptors.3 SRAP physically interacts with multiple transcription factors and is recruited to specific promoter regions. Artificially recruiting SRAP to the promoter of a luciferase reporter gene under the control of the strong transcriptional activator VP16 leads to a decrease in transcription. It is suggested that that SRAP could be a new transcriptional regulator, able to function as a repressor through direct association with promoters.4
REFERENCES
1. Lanz, R.B. et al: Mol. Cell. Biol. 23:7163-76, 2003
2. Leyque, E. et al: Cancer Res. 59:4190-3, 1999
3. Chooniedass-Kothari, S. et al: FEBS Lett 584:1174-80, 2010
4. Chooniedass-Kothari, S. et al: FEBS Lett 584:2218-24, 2010
2. Leyque, E. et al: Cancer Res. 59:4190-3, 1999
3. Chooniedass-Kothari, S. et al: FEBS Lett 584:1174-80, 2010
4. Chooniedass-Kothari, S. et al: FEBS Lett 584:2218-24, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10229 |
Antigen: | Purified recombinant human SRA1 protein fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 32 kDa |
Specificity/Sensitivity: | Detects endogenous SRA1 proteins without cross-reactivity with other related proteins |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой