Anti-SYP: Mouse Synaptophysin Antibody |
 |
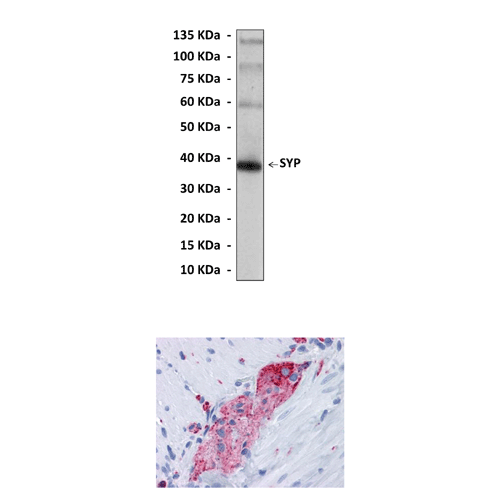
BACKGROUND The integral membrane protein synaptophysin (SYP; molecular weight, 38-kDa) is one of the most abundant transmembrane glycoproteins of synaptic vesicles. synaptophysin (SYP) is not essential for neurotransmission despite its abundance but is believed to modulate the efficiency of the synaptic vesicle cycle. It was suggested that through its association with dynamin synaptophysin (SYP) controls the fine tuning of transmitter.1 However, studies showed that synaptophysin is a probable component of neurotransmission occurring in taste receptor cells.2 Additionally synaptophysin (SYP) is well accepted as a neuroendocrine marker. It has been also used as a marker in order to study the membrane events that take place during transmitter release at the mouse neuromuscular junction (NMJ). It was shown that synaptophysin (SYP) is an immunohistochemical marker for degenerating neurons in equine grass sickness (GS). It was also detected in normal neuroendocrine cells of human adrenal medulla, carotid body, skin, pituitary gland, thyroid, lung, pancreas, gastrointestinal mucosa, Paneth’s cells in the gastrointestinal tract and of gastric parietal cells, and neurons in the brain, spinal cord, and retina. On the other hand, the expression of the SYP gene family is not restricted to neuronal and neuroendocrine differentiation in rats or humans.
Peroxynitrite is a potent oxidant that contributes to tissue damage in neurodegenerative disorders. It was shown that following peroxynitrite treatment, synaptophysin (SYP) can be both phosphorylated and nitrated in a dose-dependent manner. Tyrosine-phosphorylated, but not tyrosine-nitrated, synaptophysin (SYP) bound to the src tyrosine kinase and enhanced its catalytic activity. These effects were mediated by direct and specific binding of the SYP cytoplasmic C-terminal tail with the src homology 2 domain. Using mass spectrometry analysis, SYP C-terminal tail tyrosine residues modified by peroxynitrite were identified: one nitration site at Tyr250 and two phosphorylation sites at Tyr263 and Tyr273. Thus, it is suggested that peroxynitrite-mediated modifications of SYP may be relevant in modulating src signaling of synaptic terminal in pathophysiological conditions.3
Peroxynitrite is a potent oxidant that contributes to tissue damage in neurodegenerative disorders. It was shown that following peroxynitrite treatment, synaptophysin (SYP) can be both phosphorylated and nitrated in a dose-dependent manner. Tyrosine-phosphorylated, but not tyrosine-nitrated, synaptophysin (SYP) bound to the src tyrosine kinase and enhanced its catalytic activity. These effects were mediated by direct and specific binding of the SYP cytoplasmic C-terminal tail with the src homology 2 domain. Using mass spectrometry analysis, SYP C-terminal tail tyrosine residues modified by peroxynitrite were identified: one nitration site at Tyr250 and two phosphorylation sites at Tyr263 and Tyr273. Thus, it is suggested that peroxynitrite-mediated modifications of SYP may be relevant in modulating src signaling of synaptic terminal in pathophysiological conditions.3
REFERENCES
1. González-Jamett, A.M. et al: J. Neurosci. 30:10683-91, 2010
2. Asano-Miyoshi, M. et al: J. Mol. Histol. 40:59-70, 2009
3. Mallozzi,C. et al: J. Neurochem. 111:859-69, 2009
2. Asano-Miyoshi, M. et al: J. Mol. Histol. 40:59-70, 2009
3. Mallozzi,C. et al: J. Neurochem. 111:859-69, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10385 |
Antigen: | Raised against purified recombinant fragments of human SYP expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 38 kDa |
Specificity/Sensitivity: | Detects SYP proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой