Anti-THAP11: Mouse THAP11 Antibody |
 |
BACKGROUND The THAP proteins (Thanatos-associated protein), a novel family of cellular factors, are characterized by the presence of an evolutionarily conserved protein motif. The motif designated as the THAP domain presents striking similarities with the site-specific DNA-binding domain of Drosophila P element transposase. Analysis of the Drosophila THAP family has revealed several interesting features. One of the Drosophila THAP proteins, dorsal-interacting protein 2, was identified earlier in a two-hybrid screen with dorsal, a transcription factor from the NF-κB family. The third multi-THAP protein, designated as HIM-17, has been shown to play a critical role in chromosome segregation during meiosis by linking chromatin modification with competence to initiate meiotic recombination by double-strand breaks. In addition, several proteins containing similar or consensus THAP domain were identified in C. elegans. There are 12 distinct human proteins that contain the THAP domain (THAP 0–11). The first THAP protein characterized was the death-associated protein DAP4/P52rIPK (THAP0). Overexpression of THAP0 restored PKR activity and eIF-2α phosphorylation, thus suppressing the effects of P58IPK. THAP1 is a novel nuclear proapoptotic factor associated with promyelocytic leukemia nuclear bodies (PML NBs). THAP1 interacts with prostate-apoptosis-response-4 (Par-4) and potentates both serum withdrawal- and TNFα-induced apoptosis. An in vitro DNA site selection strategy showed that the THAP domain of THAP1 could bind directly to a specific DNA sequence. Recently, THAP1 was also identified as an endogenous physiologic regulator of endothelial cell proliferation and G1/S cell-cycle progression. THAP7 was characterized as a protein with both histone-binding and putative DNA-binding motifs. THAP7 repressed transcription by recruiting NCoR and HDAC3 to promoters and promoting the deacetylation of histone H3. The study also showed that THAP7 possessed additional mechanisms of repressing transcription by recruiting the INHAT subunit TAF-Iβ to promoters and masking histone acetylation. These studies, together with the data obtained in C. elegans, suggested that THAP proteins are sequence-specific DNA-binding factors involved in cell proliferation, apoptosis, chromatin modification and transcriptional regulation.1
THAP11 maps to a locus on chromosome 16q22.1 and was suggested as a putative candidate for polyglutamine disorders based on polymorphism and protein-folding simulation studies. Most recently, the mouse homolog of THAP11, Ronin, was identified as an essential factor underlying embryogenesis and ES cell pluripotency, and was associated with sequence-specific DNA binding and epigenetic silencing of several gene expressions. It was demonstrated that THAP11 suppresses cell growth through downregulation of c-Myc.2 Moreover, THAP11 could make chromatin more condensed and repress transcription. It was shown that THAP11 may be a tumor-suppressor.3
THAP11 maps to a locus on chromosome 16q22.1 and was suggested as a putative candidate for polyglutamine disorders based on polymorphism and protein-folding simulation studies. Most recently, the mouse homolog of THAP11, Ronin, was identified as an essential factor underlying embryogenesis and ES cell pluripotency, and was associated with sequence-specific DNA binding and epigenetic silencing of several gene expressions. It was demonstrated that THAP11 suppresses cell growth through downregulation of c-Myc.2 Moreover, THAP11 could make chromatin more condensed and repress transcription. It was shown that THAP11 may be a tumor-suppressor.3
REFERENCES
1. Roussigne, M. et al: Trends Biochem Sci. 28:66-9, 2003
2. Zhu, C.Y. et al: Cell death Differ. 16:395-405, 2009
3. Moloney, F.J. et al: J. Invest. Dermatol. 129:1012-9, 2009
2. Zhu, C.Y. et al: Cell death Differ. 16:395-405, 2009
3. Moloney, F.J. et al: J. Invest. Dermatol. 129:1012-9, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10274 |
Antigen: | Purified recombinant human THAP11 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
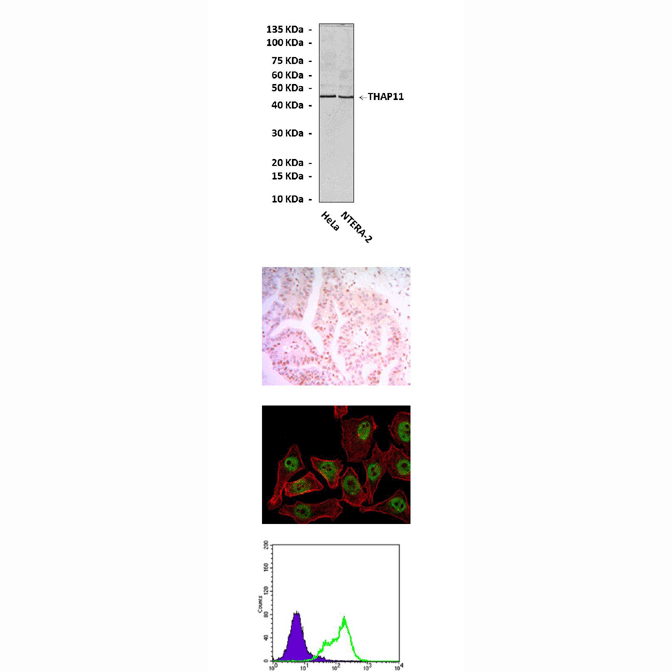
Predicted Molecular Weight of protein: | 45 kDa |
Specificity/Sensitivity: | Detects THAP11 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой