Anti-TIMP-2: Polyclonal Tissue Inhibitors of Metalloproteinases-2 Antibody |
 |
BACKGROUND The TIMPs are well-studied inhibitors of MMPs and consist of a family of four structurally related proteins (TIMP-1–4), with core proteins of ∼21 kDa. TIMPs inhibit MMP activity by a common mechanism involving interaction of the amino-terminal cysteine residue with the zinc atom at the MMP active site. The TIMPs inhibit MMP activity associated with tumor invasion and angiogenesis. In addition to their MMP-inhibitory activity, it is now widely appreciated that TIMPs have direct effects on cellular behaviors such as cell growth, apoptosis, migration, and differentiation.1
TIMP-2 is a nonglycosylated 194 amino acid protein of 21 kDa molecular mass. It shares 40% amino acid sequence homology with TIMP1 especially in the N-terminal domain. TIMP2 complexes with some of enzymes of metalloproteinases family and irreversibly inactivates them. It is known to act on MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-13, MMP-14, MMP-15, MMP-16 and MMP-19. However, TIMP2 is a positive regulator of MMP-14 (MT1-MMP) by promoting the availability of the enzyme at the cell surface and supporting pericellular proteolysis (after forming the trimolecular complex of MMP-14, TIMP-2 and proMMP-2). Through this activity of TIMP2 the specific activation of proMMP-2, after the interaction of TIMP2 with MT1-MMP (possibly MT2-MMP and MT3-MMP) in cell surface, is achieved. TIMP2 has been found to block tumor cell invasion both in vitro and in vivo and may act as metastasis suppressor gene.2 However, TIMP-2 promotes the proliferation of some cell types and its anti-apoptotic effect may favor tumor expansion during the onset and early primary tumor growth.
It was demonstrated that TIMP-2 can inhibit the proliferation of endothelial cells in response to angiogenic stimuli, such as fibroblast growth factor 2 or VEGF-A. TIMP-2 inhibits FGF-2 signaling pathways through association with integrin alpha3beta1 and Shp-1-dependent inhibition of p42/44MAPK signaling, which in turn, results in suppression of FGF-2-stimulated endothelial cell mitogenesis.3 Moreover, TIMP-2 treatment of human microvascular endothelial cells (hMVECs) results in the reduction of Src kinase activity and dephosphorylation of paxillin at Tyr-31/118. Such TIMP-2 effects accompany the disassembly of paxillin-Crk-DOCK180 molecular complex and, in turn, Rac1 inactivation. On the contrary, TIMP-2 also promotes the association of Crk with C3G, a guanine nucleotide exchange factor (GEF) of Rap1, and subsequently rap1 activation, which leads to the enhanced RECK gene expression and suppression of endothelial cell migration.4
TIMP-2 is a nonglycosylated 194 amino acid protein of 21 kDa molecular mass. It shares 40% amino acid sequence homology with TIMP1 especially in the N-terminal domain. TIMP2 complexes with some of enzymes of metalloproteinases family and irreversibly inactivates them. It is known to act on MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-13, MMP-14, MMP-15, MMP-16 and MMP-19. However, TIMP2 is a positive regulator of MMP-14 (MT1-MMP) by promoting the availability of the enzyme at the cell surface and supporting pericellular proteolysis (after forming the trimolecular complex of MMP-14, TIMP-2 and proMMP-2). Through this activity of TIMP2 the specific activation of proMMP-2, after the interaction of TIMP2 with MT1-MMP (possibly MT2-MMP and MT3-MMP) in cell surface, is achieved. TIMP2 has been found to block tumor cell invasion both in vitro and in vivo and may act as metastasis suppressor gene.2 However, TIMP-2 promotes the proliferation of some cell types and its anti-apoptotic effect may favor tumor expansion during the onset and early primary tumor growth.
It was demonstrated that TIMP-2 can inhibit the proliferation of endothelial cells in response to angiogenic stimuli, such as fibroblast growth factor 2 or VEGF-A. TIMP-2 inhibits FGF-2 signaling pathways through association with integrin alpha3beta1 and Shp-1-dependent inhibition of p42/44MAPK signaling, which in turn, results in suppression of FGF-2-stimulated endothelial cell mitogenesis.3 Moreover, TIMP-2 treatment of human microvascular endothelial cells (hMVECs) results in the reduction of Src kinase activity and dephosphorylation of paxillin at Tyr-31/118. Such TIMP-2 effects accompany the disassembly of paxillin-Crk-DOCK180 molecular complex and, in turn, Rac1 inactivation. On the contrary, TIMP-2 also promotes the association of Crk with C3G, a guanine nucleotide exchange factor (GEF) of Rap1, and subsequently rap1 activation, which leads to the enhanced RECK gene expression and suppression of endothelial cell migration.4
REFERENCES
1. Gomez, D.E.: Eur J Cell Biol. 74:111-22, 1997
2. Jezierska, A. & Motyl, T.: Med. Sci. Monit. 15:RA32-40, 2009
3. Seo, D.W. et al: Microvasc. Res. 76(3):145–51, 2008
4. Oh, J. et al: Oncogene 25:4230-4, 2006
2. Jezierska, A. & Motyl, T.: Med. Sci. Monit. 15:RA32-40, 2009
3. Seo, D.W. et al: Microvasc. Res. 76(3):145–51, 2008
4. Oh, J. et al: Oncogene 25:4230-4, 2006
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA0576 |
Antigen: | C- terminal sequence of human TIMP-2 |
Isotype: | Affinity-Purified Rabbit Polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500 to 1:1000 IP n/d IHC (Paraffin) 1:50 to 1:200 ICC n/d FACS n/d |
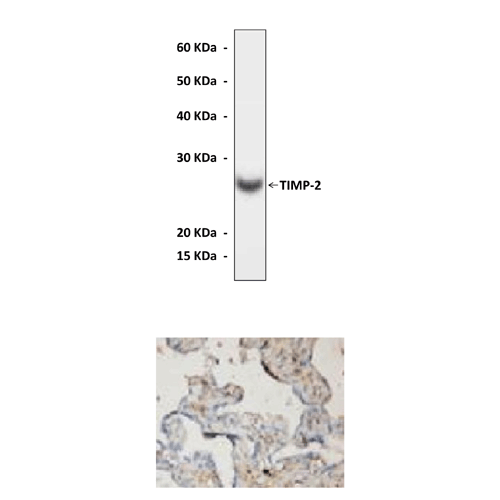
Predicted Molecular Weight of protein: | 26 kDa |
Specificity/Sensitivity: | Anti-TIMP-2 reacts specifically with TIMP-2 of human, rabbit, mouse & rat origin in immunostaining and western blotting, no cross-reactivity with other members of the MMP family. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой