MMP-2 ELISA Kit, Rat |
 |
BACKGROUND The Matrix Metalloproteinases (MMPs) are a family of zinc-containing endopeptidases that play a key role in both physiological and pathological tissue remodeling. 21 different human MMPs have been identified. These enzymes are classified according to their structure and substrate specificity into four major subgroups: the interstitial collagenases, gelatinases, stromelysins and membrane-type MMPs (MT-MMPs). MMPs regulate a variety of normal cell functions and host defense mechanisms. They are also associated with the tumorigenic process. MMPs degrade the extracellular matrix, promoting tumor invasion and metastasis.1
MMP-2 (gelatinase A), which is also produced by human endometrial stromal cells, is an unusual MMP in that its expression is not regulated by the same array of steroid hormones, cytokines, and growth factors as are the other secreted MMPs. MMP-2 expression is dependent on extracellular matrix metalloproteinase inducer (EMMPRIN), Her2/neu, growth factors, cytokines, and hormones. Furthermore, proMMP-2 is ubiquitously released by many types of cells and can be detected in plasma. Its major substrates are collagens type IV, V, and VII, elastin, fibronectin, laminin-5, and the core proteins of proteoglycans. In addition, MMP-2 can process human interleukin-1β precursor into biologically active forms. MMP-2 is also an exception within the MMP family in that it cannot be activated by serine proteinases such as plasmin or by catalytic quantities of other MMPs. However, at least in tumor cells, proMMP-2 is uniquely activated by the membrane-type (MT)-MMPs, which localize the activation to the surface of those cells expressing the enzymes. It has been suggested that cellular activation of proMMP-2 may be initiated by different members of this MT-MMP family depending on tissue distribution. This activation mechanism involves also the tissue inhibitor of Metalloproteinase (TIMP)-2. Different mechanisms may be involved in the activation of MMP-2 produced by normal fibroblasts.2
MMP-2 is an enzyme that degrades components of the extracellular matrix and thus plays a pivotal role in cell migration, tissue remodeling, and tumor metastasis (e.g. gastric, pancrcreatic, prostate, and breast cancer). The active forms of MMPs subsequently release a cascade of activation of the remaining pro-MMPs. Inactivation of the physiological function of MMPs, or even pro-MMPs, is accomplished by non-covalent TIMP binding. The detection of active MMP-2 alone or the rate of pro-MMP-2 and active MMP-2 is considered a very sensitive indicator of cancer metastasis. Modulation of MMP-2 expression and activation through specific inhibitors and activators may thus provide a new mechanism for cancer treatment. Degradation of the cellular network established by adhesion molecules such as E-cadherin or ALCAM/CD166 causes tumor tissue relaxation, increases metastasis, and correlates with shortened survival in patients with primary carcinoma. A low level of MMP-2 is linked to favorable prognosis in patients with a hormone receptor-negative tumor, usually associated with high risk. Blocking MMP-2 secretion and activation during carcinoma development may decrease metastasis. Furthermore, it was shown that modified synthetic siRNA targeting TIMP-2 may also regulate the balance between MMPs and TIMP-2 and thus decrease the degradation of extracellular matrix and prevent distant metastasis.3
MMP-2 (gelatinase A), which is also produced by human endometrial stromal cells, is an unusual MMP in that its expression is not regulated by the same array of steroid hormones, cytokines, and growth factors as are the other secreted MMPs. MMP-2 expression is dependent on extracellular matrix metalloproteinase inducer (EMMPRIN), Her2/neu, growth factors, cytokines, and hormones. Furthermore, proMMP-2 is ubiquitously released by many types of cells and can be detected in plasma. Its major substrates are collagens type IV, V, and VII, elastin, fibronectin, laminin-5, and the core proteins of proteoglycans. In addition, MMP-2 can process human interleukin-1β precursor into biologically active forms. MMP-2 is also an exception within the MMP family in that it cannot be activated by serine proteinases such as plasmin or by catalytic quantities of other MMPs. However, at least in tumor cells, proMMP-2 is uniquely activated by the membrane-type (MT)-MMPs, which localize the activation to the surface of those cells expressing the enzymes. It has been suggested that cellular activation of proMMP-2 may be initiated by different members of this MT-MMP family depending on tissue distribution. This activation mechanism involves also the tissue inhibitor of Metalloproteinase (TIMP)-2. Different mechanisms may be involved in the activation of MMP-2 produced by normal fibroblasts.2
MMP-2 is an enzyme that degrades components of the extracellular matrix and thus plays a pivotal role in cell migration, tissue remodeling, and tumor metastasis (e.g. gastric, pancrcreatic, prostate, and breast cancer). The active forms of MMPs subsequently release a cascade of activation of the remaining pro-MMPs. Inactivation of the physiological function of MMPs, or even pro-MMPs, is accomplished by non-covalent TIMP binding. The detection of active MMP-2 alone or the rate of pro-MMP-2 and active MMP-2 is considered a very sensitive indicator of cancer metastasis. Modulation of MMP-2 expression and activation through specific inhibitors and activators may thus provide a new mechanism for cancer treatment. Degradation of the cellular network established by adhesion molecules such as E-cadherin or ALCAM/CD166 causes tumor tissue relaxation, increases metastasis, and correlates with shortened survival in patients with primary carcinoma. A low level of MMP-2 is linked to favorable prognosis in patients with a hormone receptor-negative tumor, usually associated with high risk. Blocking MMP-2 secretion and activation during carcinoma development may decrease metastasis. Furthermore, it was shown that modified synthetic siRNA targeting TIMP-2 may also regulate the balance between MMPs and TIMP-2 and thus decrease the degradation of extracellular matrix and prevent distant metastasis.3
REFERENCES
1. Malemud, C.J.: Front.in Biosci. 11:1696-701, 2006
2. Oh, J. et al: Oncogene 23:5041-8, 2004
3. Sierevogel, M.J. et al: 9: Curr. Pharm. Desig.1033-40, 2003
2. Oh, J. et al: Oncogene 23:5041-8, 2004
3. Sierevogel, M.J. et al: 9: Curr. Pharm. Desig.1033-40, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0639 |
Target Protein Species: | Rat |
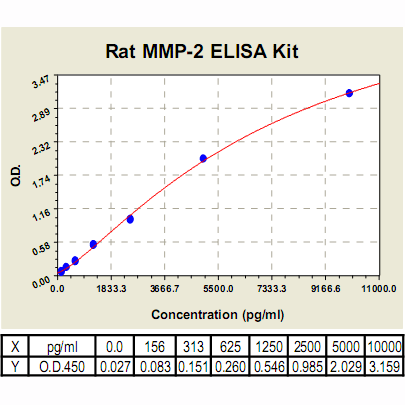
Range: | 156 pg/ml – 10000pg/ml |
Specificity: | No detectable cross-reactivity with other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой