The intestinal epithelium is a single layer of cells organized into crypts and villi, known as the most rapidly self-renewing tissue in adult mammals. The cells that line the intestinal lumen perform the primary functions of digestion, water and nutrient absorption, and forms a barrier against luminal pathogens.
Transit-amplifying cells spend approximately two days in the intestinal crypts, dividing 4–5 times before terminally differentiating into specialized intestinal epithelial cell types. In the small intestine, the surface area is dramatically enlarged through epithelial protrusions called villi. Three days after their terminal differentiation, the cells reach the tip of the villus, undergo spontaneous apoptosis, and are shed into the gut lumen.
CAI’s intestinal epithelial culture system provides outstanding resource for investigation of intestinal epithelial cell physiology related to GI infection, inflammatory bowel disease (IBD) like Crohn’s disease, ulcerative colitis, and intestinal cancer. Our epithelial cell culture system can be efficiently used as a test platform for the potential drug candidates and disease modulators. Other applications of this culture system include functional analysis of intestinal epithelium, GI disease modeling, and regenerative therapy preclinical testing such as drug compound screening and other validation assays.
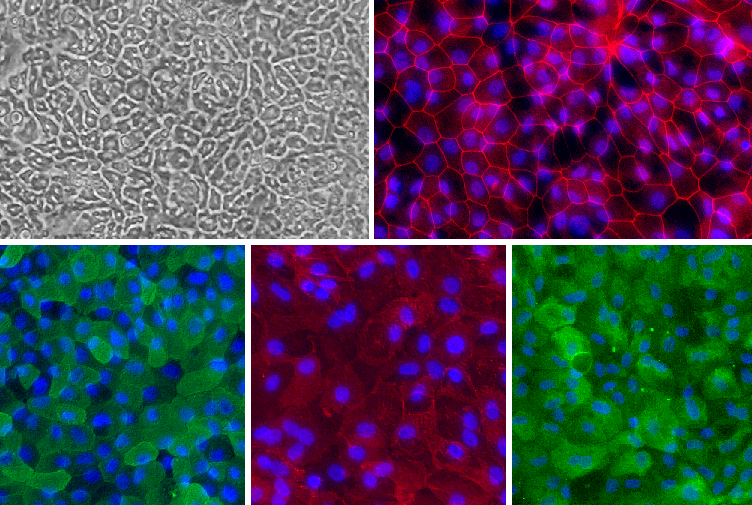
With optimized, defined culture media from CAI, the Intestinal Epithelial Cells can be seeded and maintained for as long as 8 days. Epithelial Cells grown in CAI medium form a monolayer of polarized epithelial cells with tight junction formation as evidenced by Villin (apical marker), Na+/K+ ATPase (basolateral marker), ZO-1 (tight junction marker) and pan-Cytokeratin (epithelial marker) staining.